The initial evaluation of persons with hepatitis B virus (HBV) should begin by confirming chronic HBV infection. Chronic infection is characterized by the presence of a positive hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (anti-HBc), with or without a positive hepatitis B DNA level. Patients with an isolated anti-HBc, or those who are both anti-HBc and hepatitis B surface antibody (anti-HBs) positive, have evidence of prior infection but generally not chronic infection.[1,2,3] In addition to confirming chronic infection, the medical provider should perform a detailed medical history and physical examination, as outlined below. A laboratory workup aimed at determining the phase of HBV infection, establishing the severity of disease, assessing for indications for treatment, and evaluating the need for liver cancer screening, should also be performed.[4,5]
- Module 3 Overview
Screening and Diagnosis - 0%Lesson 1
HBV EpidemiologyLesson 3. Initial Evaluation of Persons with Chronic Hepatitis B
Learning Objective Performance Indicators
- Summarize key aspects of the medical history and physical examination in persons with hepatitis B who are presenting for an initial evaluation
- Describe the initial laboratory workup for persons with chronic hepatitis B
- List key immunizations that should be administered to persons with chronic hepatitis B infection
- Summarize noninfectious causes of liver disease that may coexist with hepatitis B
Last Updated: December 30th, 2024Authors:Maria A. Corcorran, MD, MPH,Maria A. Corcorran, MD, MPH
Assistant Professor
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneH. Nina Kim, MD, MScH. Nina Kim, MD, MSc
Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneReviewer:David H. Spach, MDDavid H. Spach, MD
Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneTable of Contents- Initial Evaluation of Persons with Chronic Hepatitis B
- General Approach to the Initial Evaluation
- Key Aspects of Medical History
- Key Aspects of the Physical Examination
- Initial Laboratory Evaluation
- Screening for Other Causes of Liver Disease
- Evaluation of Fibrosis Stage
- Immunizations for Persons with Chronic HBV and Cirrhosis
- Counseling Persons with Chronic HBV Following Diagnosis
- Summary Points
- Citations
- Additional References
- Figures
- Tables
General Approach to the Initial Evaluation
Key Aspects of Medical History
In addition to the standard medical history, the initial history of patients with chronic hepatitis B should focus on risk factors for HBV, including family history of HBV or liver disease, history of alcohol use, any prior evaluation for cirrhosis or hepatocellular carcinoma (HCC), prior treatment of HBV, presence of advanced liver disease, history of extrahepatic manifestations of HBV, co-occurring viral infections, and other key noninfectious comorbidities.
Identifying Risk Factors for HBV
A key aspect of the initial evaluation of persons with chronic HBV is to identify risk factors for HBV acquisition. This information enables the clinician to better assess the duration of infection, determine the risk of advanced liver disease, and provide counseling regarding prevention of HBV transmission to others. Important elements of the initial history include prior or current injection drug use, history of multiple sex partners, prior exposure to blood or bodily fluids (including occupational exposure), and family history of HBV or liver disease.[5] At the initial intake, some individuals may be reluctant to disclose a history of remote injection drug use or multiple sex partners. Care should be taken to establish rapport and a safe, nonjudgmental environment to facilitate this discussion. In the United States, injection drug use and sex with multiple partners are the most important risk factors for acquiring HBV.[6] Globally, the majority of chronic HBV infections are acquired before the age of 5 years, with perinatal transmission being the most common mode of HBV acquisition, especially in countries with high HBV endemicity.[7,8]
Table 1. Global Prevalence of Chronic HBV Infection, by Country
Prevalence Category Country High
(≥8%)Angola, Cabo Verde, Central African Republic, Chad, Eswatini, Ghana, Guinea, Guinea-Bissau, Kiribati, Lesotho, Liberia, Mali, Mauritania, Niger, Nigeria, Philippines, Sao Tome and Principe, Sierra Leone, Solomon Islands, Taiwan, Timor-Leste, Togo, Tonga, Turkmenistan, Tuvalu, and Zimbabwe. Intermediate
(5.0-7.9%)Albania, Benin, Burkina Faso, Cameroon, China, Côte d’Ivoire, Democratic People’s Republic of Korea, Djibouti, Eritrea, Ethiopia, Federated States of Micronesia, Gabon, Indonesia, Kyrgyzstan, Moldova, Mongolia, Mozambique, Myanmar, Papua New Guinea, Senegal, Somalia, South Sudan, Syria, Tajikistan, Uzbekistan, Vanuatu, and Vietnam. Low Intermediate
(2.0-4.9%)Afghanistan, Azerbaijan, Bangladesh, Belarus, Bosnia and Herzegovina, Bulgaria, Burundi, Cambodia, Comoros, Congo, Democratic Republic of Congo, Gambia, Georgia, Guyana, Haiti, Hong Kong, India, Iraq, Jamaica, Jordan, Kazakhstan, South Korea, Laos, Madagascar, Malawi, Malaysia, Marshall Islands, Oman, Pakistan, Romania, Rwanda, Samoa, Singapore, South Africa, Sri Lanka, Sudan, Tanzania, Thailand, Trinidad and Tobago, Tunisia, Turkey, Uganda, Yemen, and Zambia. Low
(≤1.9%)Algeria, Argentina, Armenia, Australia, Austria, Bahrain, Belgium, Belize, Bhutan, Bolivia, Brazil, Canada, Chile, Colombia, Costa Rica, Croatia, Cuba, Czechia, Denmark, Dominican Republic, Ecuador, Egypt, El Salvador, Estonia, Fiji, Finland, France, Germany, Greece, Guatemala, Honduras, Hungary, Iran, Ireland, Israel, Italy, Japan, Kenya, Kosovo, Kuwait, Lebanon, Libya, Mexico, Morocco, Nepal, Netherlands, New Zealand, Nicaragua, Norway, Palestine, Panama, Paraguay, Peru, Poland, Portugal, Qatar, Russia, Saudi Arabia, Slovakia, Slovenia, Spain, Suriname, Sweden, Switzerland, Ukraine, United Arab Emirates, United Kingdom, United States, and Venezuela. Unknown prevalence (data not available)
American Samoa, Andorra, Anguilla, Antigua and Barbuda, Aruba, Bahamas, Barbados, Bermuda, Bonaire Sint Eustatius and Saba, Botswana, British Virgin Islands, Brunei, Cayman Islands, Cook Islands, Curaçao, Cyprus, Dominica, Equatorial Guinea, Falkland Islands, Faroe Islands, French Guiana, French Polynesia, Gibraltar, Greenland, Grenada, Guadeloupe, Guam, Holy See, Iceland, Isle of Man, Latvia, Liechtenstein, Lithuania, Luxembourg, Macao, Macedonia, Maldives, Malta, Martinique, Mauritius, Mayotte, Monaco, Montenegro, Montserrat, Namibia, Nauru, New Caledonia, Niue, Northern Mariana Islands, Palau, Puerto Rico, Réunion, Saint Barthélemy, Saint Helena, Saint Kitts and Nevis, Saint Lucia, Saint Martin, Saint Pierre and Miquelon, Saint Vincent and the Grenadines, San Marino, Serbia, Seychelles, Sint Maarten, Tokelau, Turks and Caicos Islands, U.S. Virgin Islands, Uruguay, Wallis and Futuna, and Western Sahara. NOTE: This table is based on data from the Centers for Disease Control and Prevention (CDC) Source:- Conners EE, Panagiotakopoulos L, Hofmeister MG, et al. Screening and Testing for Hepatitis B Virus Infection: CDC Recommendations - United States, 2023. MMWR Recomm Rep. 2023;72:1-25. [PubMed Abstract]
History of Alcohol Use
Due to its deleterious effects on the liver, it is important to inquire about alcohol use among persons with chronic HBV. Although there is no clear guidance on what quantity of alcohol may be safe in persons with chronic HBV infection, studies suggest heavy alcohol use in persons with HBV can increase the risk of HCC 1.3- to 8.4-fold, in comparison to individuals with chronic HBV who are not heavy drinkers.[9,10,11,12] Accordingly, persons with chronic HBV should be counseled to avoid alcohol and be directed to educational resources, such as the National Institute on Alcohol Abuse and Alcoholism (NIAAA) site Rethinking Drinking (Alcohol and Your Health).[13,14] In clinical practice, it can often be challenging to obtain an accurate history of alcohol consumption. A general approach would be to assess the quantity of use over a specified period of time without using pejorative qualifiers such as “heavy” or “excessive” in the inquiry. Several well-validated screening tools, as outlined below, are available to help assess for alcohol use disorder.
- CAGE: The CAGE is a 4-question screening tool for alcohol use disorder that focuses on Cutting down, Annoyance by criticism, Guilty feeling, and Eye-openers (see CAGE screening tool).[15]
- AUDIT-C: A shorter 3-question version of the AUDIT, known as the AUDIT-C, has also been validated and performs similarly to the AUDIT for detecting heavy drinking and/or alcohol dependence (see AUDIT-C screening tool).[16]
- AUDIT: The AUDIT, or Alcohol Use Disorder Identification Test, is a 10-item questionnaire that can be used to screen for hazardous drinking.[17]
Prior Evaluation for Cirrhosis
Cirrhosis is a key predictor of liver-related complications such as HCC and is an indication for HCC screening. In addition, treatment of chronic HBV is indicated for all persons with cirrhosis.[13,18] As such, among persons who have previously been engaged in HBV care, it is important to understand if they have undergone a prior evaluation for cirrhosis and what that evaluation revealed. Although guidelines do not specify the best method to diagnose cirrhosis in persons with chronic HBV, a variety of modalities are currently used, including liver biopsy, hepatic ultrasound, transient elastography, laboratory markers, and clinical examination. Understanding the results and timing of prior fibrosis assessments can inform the need for antiviral therapy and help triage the need for additional fibrosis assessment. A more detailed discussion on evaluating for cirrhosis in persons with chronic HBV can be found in the lesson When to Initiate HBV Treatment.
Prior Screening for Hepatocellular Carcinoma (HCC)
Given the oncogenic properties of HBV and the elevated risk of developing hepatocellular carcinoma (HCC) among people with chronic HBV, it is also important to obtain information on prior screening for HCC, typically in the form of an abdominal ultrasound. Multiphase imaging studies, such as computed tomography (CT) and magnetic resonance imaging (MRI), are also used in some circumstances.[13,19] A more detailed discussion on this topic can be found in the lesson Screening for Hepatocellular Carcinoma.
Prior or Current Antiviral Therapy for HBV
When evaluating persons with chronic HBV, clinicians should inquire about prior or current antiviral therapy for HBV, including the type of treatment, duration of therapy, response to therapy, level of adherence, treatment-related adverse effects, and reasons for stopping therapy (if discontinued). For persons with HIV and chronic HBV coinfection, this would entail a detailed overview of past antiretroviral therapy because the nucleos(t)ide analogues emtricitabine, lamivudine, tenofovir alafenamide, or tenofovir DF are also active against HBV. Information on past treatment regimens can help determine the risk of drug resistance and guide recommendations for future treatment. This is particularly the case for lamivudine and emtricitabine, which have been and continue to be a common component of HIV treatment regimens and have a notably low barrier to HBV resistance when used as the sole HBV-active agent.[20,21]
Presence of Advanced Liver Disease
The management of persons with advanced liver disease can be complex. As discussed above, it is important to understand what, if any, prior liver disease staging the patient has undergone (e.g., liver biopsy). In addition to assessing for a history of cirrhosis, it is important to assess for signs and symptoms of decompensated cirrhosis, including current or past ascites, hepatic encephalopathy, jaundice, scleral icterus, and gastrointestinal bleeding. If advanced liver disease is suspected, the clinician can calculate the Child-Turcotte-Pugh (CTP) stage to help estimate the severity of cirrhosis (see CTP Calculator). Persons with chronic HBV who are classified as CTP stage B or C have decompensated cirrhosis and should be urgently referred to a liver specialist.
Presence of Extrahepatic Manifestations
Hepatitis B can be associated with a variety of extrahepatic manifestations, most of which are immune-mediated.[22] The most clinically significant extrahepatic manifestations include a non-erosive arthritis that can be mono- or polyarticular in distribution, polyarteritis nodosa (a type of vasculitis with potential for skin, gastrointestinal tract, and joint involvement), glomerular disease (commonly membranous nephropathy or membranoproliferative glomerulonephritis), and a serum sickness-like reaction that can occur during acute HBV infection.[23,24,25,26]
Key Viral Coinfections
During the initial evaluation of persons with chronic hepatitis B, it is important to evaluate for other viral infections, such as human immunodeficiency virus (HIV), HCV, hepatitis A virus (HAV), and possibly hepatitis D virus (HDV).
- HAV: Acute HAV infection can result in a more severe clinical course, including fulminant liver failure, in patients with chronic HBV.[27] Screening for HAV is important since there is a highly effective vaccine to prevent HAV infection.
- HCV: Coinfection with HCV can accelerate progression of liver disease in persons with chronic HBV.[28] Evaluation for HCV is especially important since there is highly effective and well-tolerated treatment for HCV.
- Hepatitis D Virus (HDV): HDV is a unique satellite virus that requires HBsAg to replicate and can therefore only occur in the presence of chronic HBV infection.[29] Coinfection with HDV can accelerate the progression of liver disease and increase the risk of developing HCC.[13] The American Association for the Study of the Liver Diseases (AASLD) guidelines recommend screening for HDV in key populations at highest risk, including those from regions of high HDV endemicity (Figure 1), persons with a history of injection drug use, men who have sex with men (MSM), individuals coinfected with HCV or HIV, persons with multiple sex partners or any history of sexually transmitted diseases, and those with persistently elevated liver enzymes despite low or undetectable HBV DNA levels.[13,30]
- HIV: Identifying persons with HIV and HBV coinfection is of particular importance, as coinfection has been shown to accelerate progression of liver disease and increase liver-associated mortality, and HIV and HBV share modes of transmission.[31] In addition, there is considerable overlap in the oral antivirals used to treat HBV and HIV, and monotherapy for HBV has resulted in the emergence of HIV drug resistance when used in the absence of an appropriate antiretroviral regimen.[32] Therefore, assessing the individual’s HIV status is critical before initiating treatment for HBV.[20,33]
Table 2. Key Characteristics of Oral Antiviral Agents Used to Treat HBV and/or HIV*
Hepatitis B Virus HIV Medication Potency Against HBV Barrier to HBV Resistance Potency Against HIV Barrier to HIV Resistance Adefovir Low Moderate Weak Low Entecavir High High Weak Low Lamivudine Moderate Low Medium Low Tenofovir alafenamide High High Medium Medium Tenofovir DF High High Medium Medium *Telbivudine is not included as it is no longer manufactured in the United States
Noninfectious Comorbidities
When evaluating persons with chronic HBV infection, the clinician should inquire about any secondary causes of liver disease, such as metabolic dysfunction-associated steatotic liver disease (MASLD), alcoholic hepatitis, alpha-1 antitrypsin deficiency, hemochromatosis, or autoimmune hepatitis.[34,35,36,37,38] A past or current history of obesity is important to obtain since obesity is strongly associated with the development of MASLD.[39]
Key Aspects of the Physical Examination
Physical Examination of a Patient with HBV Infection
During the initial evaluation visit, the clinician should ideally perform a complete physical examination, including obtaining the patient's height and weight to determine the body mass index (BMI). The calculation of an individual's BMI is based on their weight (pounds) and height (inches) (BMI Calculator) (Figure 2). The National Heart, Lung, and Blood Institute has BMI Tables for interpreting the calculated BMI result. In addition, there are several liver-related physical findings that should be specifically sought and may suggest the presence of advanced liver disease.
Physical Examination Findings in Patients with Cirrhosis
The following shows illustrations and descriptions of key physical examination findings that may indicate the presence of cirrhosis (Figure 3).[40,41,42,43] It is worth noting that none of these are sufficiently sensitive findings for the presence of liver disease; the absence of any one or a combination of these does not rule out the possibility of cirrhosis or portal hypertension.
- Ascites: The term ascites refers to an abnormal accumulation of fluid in the abdominal cavity. It is the most common initial complication of cirrhosis, with approximately 50% of patients with compensated cirrhosis developing ascites over a 10-year period. The presence of bulging flanks and/or flank dullness suggests the presence of ascites.[44] In order for the flank dullness to be appreciated on physical examination, at least 1,500 mL of fluid needs to be present. The shifting dullness test improves the diagnostic sensitivity of physical examination for detecting the presence of ascites.[44]
- Distended Abdominal Veins and Caput Medusae: If a patient with cirrhosis develops portal hypertension, the increased pressure can cause swelling of the collateral venous channels, which may become evident as distended abdominal veins. The distended abdominal veins can radiate around the umbilicus, a finding referred to as caput medusae.[45,46] On general inspection, the cirrhosis-related abdominal vein swelling can appear similar to findings with obstructions of the inferior vena cava.
- Gynecomastia: The presence of true gynecomastia refers to enlargement of the male breast glandular tissue and should be distinguished from generalized breast enlargement from fat accumulation in the breast region (lipomastia), which may be associated with obesity.[47] Cirrhosis-related gynecomastia results from impaired hepatic degradation of estrogens, a problem enhanced in persons with excess alcohol consumption (because of the phytoestrogens in alcohol). The finding of gynecomastia is not specific to cirrhosis and can also be seen as a side effect of medications, including spironolactone.[47,48]
- Jaundice : The term jaundice refers to a yellow discoloration of the skin or sclera that results from excess deposition of biliary pigments. The sclera and mucous membranes under the tongue are the most sensitive sites to detect jaundice.[49] Jaundice is usually only detected when the serum bilirubin level exceeds 2.5 mg/dL. In one study, 58% of clinicians were able to detect scleral icterus when the serum bilirubin was 2.5 mg/dL and 68% when the serum bilirubin was 3.1 mg/dL.[49] The finding of jaundice is often an indicator of advanced liver disease, and in persons with chronic liver disease it strongly suggests decompensated cirrhosis. Jaundice can also result from nonhepatic causes, such as hemolytic anemia.
- Palmar Erythema: The finding of palmar erythema is suggested by the presence of intense erythema in the thenar and hypothenar eminence (base of the thumb and fifth finger) of the palm, with the central region of the palm spared.[50] Approximately 25% of persons with cirrhosis have palmar erythema. This finding is not specific to cirrhosis and can be seen in pregnant women, thyrotoxicosis, and rheumatoid arthritis.[42]
- Spider Nevi (Spider Angiomata): This finding results from dilated arterial blood vessels found just below the skin surface. The lesion is referred to as a spider nevus because of the appearance of the central arteriole that has multiple thin-walled radiating blood vessels that resemble spider legs.[51] With direct compression on the central region of the lesion, the lesion will temporarily blanch, but with the release of pressure the lesion fills back in from the center, radiating outward. Typically, the presence of more than three spider nevi is considered abnormal, but this finding is not specific to liver disease. In persons with cirrhosis, elevated levels of vascular endothelial growth factor (VEGF), basic fibroblastic growth factor (bFGF), and substance P are thought to play a role in the development of spider angioma.[52]
- Splenomegaly: To increase the likelihood of palpating the spleen, have the patient lay on their right side and flex their legs towards their body. The detection of splenomegaly on physical examination suggests cirrhosis and portal hypertension, although this finding is not specific for liver disease.[43]
- Terry's Nails: The initial finding of Terry's nails consists of a white-silver discoloration of the proximal nail bed, typically with a pink band on the distal portion of the nail bed; as this process progresses, the white discoloration can involve about 80% of the nail bed, with only a 0.5 to 3.0 mm pink band remaining on the distal nail plate.[53,54] This finding can be distinguished from onychomycosis, since Terry's nails involve the nail bed and have a pink-brown band, whereas onychomycosis involves the nail itself, without any pink distal band.
Accuracy of Physical Examination for Detecting Cirrhosis
Although cirrhosis is ultimately a histological diagnosis, several clinical signs and symptoms strongly suggest the presence of cirrhosis. In a meta-analysis of 86 studies, Udell and coworkers found that specific physical examination findings increase the likelihood a patient has cirrhosis: distended abdominal veins, encephalopathy, ascites, and spider nevi (all with a likelihood ratio [LR] greater than 4).[43] The LR of any clinical finding is the probability of that finding in patients with disease divided by the probability of the same finding in patients without disease.[55] Although Terry's nails and gynecomastia had high likelihood ratios, the confidence intervals were broad, and the validity was thus harder to interpret. The following table is a summary for the diagnostic accuracy of the physical examination for detecting cirrhosis, in decreasing order of positive LR for the presence of cirrhosis.[43]
Table 3. Diagnostic Accuracy of the Physical Examination for Detecting Cirrhosis
Finding Sensitivity Specificity Positive LR (95% CI) Terry nails 0.43-0.44 0.97-0.98 16-22 Gynecomastia 0.18-0.58 0.97-0.98 5.8-35 Distended abdominal veinsa 0.31 0.98 11 (2.7-44) Encephalopathyb 0.16 0.98 10 (1.5-77) Decreased body haira 0.36 0.97 9.0 (6.4-13) Ascitesb 0.35 0.95 7.2 (2.9-12) Facial telangiectasia 0.73-0.82 0.88-0.92 5.9-10 Testicular atrophy 0.18 0.97 5,8 (2.4-14) Palmar erythemab 0.46 0.91 5.0 (0.80-9.1) Spider nevia 0.46 0.89 4.3 (2.4-6.2) Jaundicea 0.28 0.93 3.8 (2.0-7.2) Splenomegalyb 0.34 0.90 3.5 (1.8-5.2) Firm livera 0.73 0.81 3.3 (2.3-4.7) Peripheral edemaa 0.37 0.90 3.0 (1.9-4.8) Hepatomegalyb 0,74 0.69 2.4 (1.2-3.6) Obesitya 0.64 0.52 1.3 (1.1-1.6) *Abbreviations: LR = likelihood ratio; CI = confidence interval
a = Univariate random-effects summary measures because data did not converge on a bivariate solution
b = Bivariate random-effects summary measuresSource:- Udell JA, Wang CS, Tinmouth J, et al. Does this patient with liver disease have cirrhosis? JAMA. 2012;307:832-42. [PubMed Abstract]
Initial Laboratory Evaluation
The initial laboratory evaluation for patients with HBV aims to assess the phase of HBV infection, screen for common medical comorbidities, including renal disease, assess for abnormalities attributable to liver injury and fibrosis, and to screen for other co-occurring viral infections.[5,13] Because chronic infection with HBV cannot be accurately diagnosed based on a single laboratory assessment, it is also advisable to repeat HBV serologies and obtain an HBV DNA level.[5] Doing so will not only allow the provider to confirm the diagnosis of HBV, but it will also enable assessment of the immune phase of chronic HBV infection, as discussed in further detail in the lesson When to Initiate HBV Treatment.
- General Laboratory Evaluation: Complete blood count (CBC), platelet count, and chemistry panel, including serum creatinine and blood urea nitrogen. The presence of renal impairment may influence the selection or dose of antiviral therapy, as different agents are approved for use in varying stages of chronic kidney disease or may require dose adjustment.
- Hepatic Function Testing: Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total and direct bilirubin, alkaline phosphatase, serum albumin, and international normalized ratio (INR).
- Hepatitis B Serologic and DNA Testing: HBV surface antibody (anti-HBs), HBV surface antigen (HBsAg), HBV core antibody (anti-HBc), HBV E antigen (HBeAg), HBV E antibody (anti-HBe), and HBV DNA.
- HBV Genotyping and Viral Resistance Testing: These tests are not routinely recommended for individuals with chronic HBV, particularly those who are treatment naïve or not on antiviral therapy.[13]
- Laboratory Testing to Assess for Co-occurring Infections: Hepatitis A virus IgG antibody, hepatitis C virus antibody (ideally with reflexive PCR), and HIV-1/2 antigen-antibody immunoassay. In select cases, as described above, testing for antibodies to the hepatitis D virus may also be indicated.[13]
- Laboratory Screening for HCC: For individuals with chronic HBV who meet the criteria for HCC screening, the AASLD guidelines recommend abdominal ultrasound and serum alpha-fetoprotein every 6 months.[56]
Screening for Other Causes of Liver Disease
Overview of Screening for Other Causes of Liver Disease
In the course of a complete workup of an individual diagnosed with chronic HBV, the clinician should make an effort to determine whether additional causes of liver disease are present, especially in cases with significant abnormalities on liver function testing. Other causes of liver disease may coexist with HBV infection, including both hereditary and acquired conditions.[57] Identifying additional causes of liver disease in persons with chronic HBV is important since the combination of diseases may result in accelerated fibrosis progression or ongoing fibrosis progression even after treatment of HBV. An exhaustive screening laboratory work-up for all these conditions would be expensive and low-yield for most patients; however, they may be relevant in special situations, such as may occur if ALT and AST do not fully normalize with antiviral therapy. Therefore, the clinician should be familiar with some of the more important nonviral causes of hepatic inflammation.
Alcoholic Liver Disease
Chronic excessive alcohol consumption is the most common cause of liver disease in the United States. and determining alcohol intake is important in persons with chronic HBV.[58,59] On a practical basis, differentiating liver injury caused by alcohol use from that due to chronic HBV infection can be difficult, but the finding of an AST/ALT ratio of greater than 2.0 suggests alcohol-related injury, although this pattern may also be seen in advanced cirrhosis of any cause.[60,61] In addition, screening for alcohol intake as part of the medical history, as outlined above, may provide useful information on whether alcohol is a likely contributor to liver disease. Excessive alcohol use can cause acute alcoholic hepatitis, fatty liver (steatosis), and eventually cirrhosis.[59,61,62] In addition, alcohol use can accelerate HBV-associated fibrosis and increase the risk of developing hepatocellular carcinoma.[9,10,11,12] Given that no consensus exists regarding a safe level of alcohol consumption for persons with chronic HBV, most experts recommend complete abstinence from alcohol.[13] If abstinence is not an achievable goal, it is important to individualize this approach and strive for harm reduction to provide reduction in alcohol intake.
Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD)
Globally, an epidemic of chronic liver disease caused by metabolic dysfunction-associated steatotic liver disease (MASLD) has emerged due to changes in lifestyle and an increasing prevalence of obesity.[63] In the United States, the prevalence of obesity is high and the MASLD prevalence in adults is estimated at approximately 25%.[64] Tthe prevalence of the more severe form of MASLD, known as metabolic dysfunction-associated steatohepatitis (MASH), has also increased substantially.[38,63] The terms MASLD and MASH were formerly referred to as nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH).[65] Common conditions that have an established association with MASLD include obesity, dyslipidemia, type 2 diabetes mellitus, metabolic syndrome, and polycystic ovary syndrome.[66] The diagnosis of MASLD requires liver imaging or biopsy showing more than 5% hepatic steatosis along with at least one identified risk factor for cardiometabolic dysfunction: overweight (BMI ≥25 or visceral adiposity), hypertension, dysglycemia, or dyslipidemia. In addition, the diagnosis of NAFLD requires documented absence of ongoing or recent substantial alcohol ingestion.[65] The more severe liver disorder, MASH, is defined as the presence of MASLD plus inflammation and hepatocellular injury.[38] The development of MASH can result in progression to cirrhosis, liver failure, and hepatocellular cancer.[39,63]
Hemochromatosis
Hemochromatosis is defined as an excessive accumulation of iron in the liver; hemochromatosis may result from excessive blood transfusions, erythrocyte disorders, or as a hereditary condition that involves a defect in iron metabolism.[57] With hereditary hemochromatosis, the total amount of body iron accumulates over time, which is associated with increased hepatic iron that can eventually cause tissue injury and complications that can include cirrhosis, arthropathy, or diabetes (and other endocrinologic disorders).[67] Type 1 hereditary hemochromatosis is the most common and best-studied hereditary hemochromatosis variant and is caused by mutations in the human factors engineering (HFE) gene.[68] Initial diagnostic laboratory studies that can suggest but not necessarily confirm a diagnosis of hemochromatosis include elevated serum iron, elevated serum ferritin concentration, and elevated transferrin saturation.[67,68] Use of these markers can be challenging since they are often elevated in patients with chronic liver disease or hepatic injury.[69] A definitive diagnosis of hemochromatosis requires either liver biopsy with determination of iron index, or a specific battery of genetic testing. For screening purposes, most expert guidelines, including those from the American Association for the Study of Liver Diseases (AASLD), recommend using the following cutoffs when screening for iron overload: transferrin saturation greater than 45% and serum ferritin greater than 200 ng/mL (for men) and greater than 150 ng/mL (for women).[37,70,71]
Autoimmune Hepatitis
This relatively rare condition results from both genetic and host factors. The disorder is believed to result from the host losing tolerance to its own liver antigens, which leads to an immune response that includes activated immune cells, autoantibodies, interferons, and proinflammatory cytokines, which together cause hepatic inflammation.[72,73] Most experts classify autoimmune hepatitis as type 1 or type 2.[35,73] Autoimmune hepatitis type-1 is more common than type-2 and predominantly occurs in adults. Approximately 20% of people with autoimmune hepatitis type-1 will have an extrahepatic autoimmune disorder, such as autoimmune thyroid disease, arthritis, or inflammatory bowel disease.[73] Autoimmune hepatitis type-2 most often affects children, and extrahepatic autoimmune complications are common, including autoimmune thyroid disease, insulin-dependent diabetes mellitus, Addison's disease, and arthritis.[73] Clinical and laboratory characteristics of autoimmune hepatitis include itching, joint pain, hypergammaglobulinemia, and chronic elevations in aminotransferase levels. The diagnosis typically depends on positive autoantibody studies combined with compatible clinical and histologic features.[74,75] Autoantibodies commonly found in persons with autoimmune hepatitis include smooth muscle antibodies (SMA), antinuclear antibodies (ANA), antimitochondrial antibodies (AMA), liver-kidney microsomal (LKM) antibodies, and soluble liver/liver-pancreas (SLA/LP) antibodies.[76] In 2008, the International Autoimmune Hepatitis Group published revised simplified criteria for the diagnosis of autoimmune hepatitis.[76] For further details on diagnosis and management, see the 2019 AASLD Practice Guidelines on autoimmune hepatitis.[77]
Alpha-1 Antitrypsin Deficiency
This rare condition is characterized by deficiency of the alpha-1 antitrypsin enzyme, resulting in overly active proteases in the body and concomitant lung and liver destruction (emphysema and cirrhosis).[78,79] It has a genetic basis with complex inheritance and varia ble penetrance but is most prevalent in Caucasians of Scandinavian descent. In the United States and Western Europe, the prevalence of alpha-1 antitrypsin deficiency is estimated between 1 in 2,000 and 1 in 5,000 population.[79] A serum alpha-1 antitrypsin level below 11 μmol/L (80 mg/dL) should prompt specific genetic testing for the most common alpha-1 antitrypsin deficiency alleles.[34]
Evaluation of Fibrosis Stage
For persons previously engaged in clinical care for HBV, it is important to determine whether they have had prior evaluation and staging of liver fibrosis. Methods to assess liver fibrosis include serum-based aspartate aminotransferase-to-platelet ratio index (APRI), FibroTest, liver transient elastography, hepatic ultrasound, and liver biopsy.[80,81] If a liver biopsy has previously been performed, it is important to document the sample size, fibrosis score, and fibrosis scoring system used in the report, as well as the year the biopsy was performed, since remote staging results may be less relevant for clinical decision-making. For a detailed discussion on this topic, see the lesson Evaluation and Staging of Liver Fibrosis on the Hepatitis C Online website.
Immunizations for Persons with Chronic HBV and Cirrhosis
The following summarizes key vaccine recommendations for persons with chronic HBV. For more details on this topic, see the Advisory Committee for Immunization Practices (ACIP) recommendations.[82]
- Hepatitis A Vaccination: Individuals with chronic HBV are at increased risk for severe clinical manifestations of acute HAV infection, including fulminant liver failure.[27] As such, all persons with HBV should receive the two-dose hepatitis A vaccine series, which is administered at 0 and 6 months.[13,82,83] The hepatitis A vaccine has been shown to be highly immunogenic in adults, with an estimated 94 to 100% of adults 18 years of age or older achieving protective antibody levels 1 month after the first dose of vaccine, and all persons achieving protective antibody levels after the second dose.[84] Given the efficacy of HAV vaccine, post-vaccination serologic testing is not routinely recommended for persons with chronic HBV, even for individuals with chronic liver disease.[84]
Table 4. Recommended Hepatitis A Immunization for Adults
Vaccine Dosage Dosing and Route Hepatitis A Vaccines
Havrix 1440 EL.U 2-Dose Schedule: 1 mL given IM at 0 and 6-12 months Vaqta 50 U 2-Dose Schedule: 1 mL given IM at 0 and 6-18 months Combined Hepatitis A and B Vaccine
Twinrix HAV: 720 EL.U
plus
HBsAg: 20 mcgStandard 3-dose series: 1 mL given IM at 0, 1, and 6 months
Accelerated 4-dose series: 1 mL given IM on days 0, 7, and 21-30, followed by a booster dose at month 12Abbreviations: IM = intramuscular; HAV = hepatitis A virus; HBsAg = hepatitis B surface antigen
- Pneumococcal Vaccination: There are two options recommended for pneumococcal immunization for people with chronic liver disease who are 19 years of age or older: (1) a single dose of the pneumococcal 20-valent conjugate vaccine (PCV20) or (2) a single dose of pneumococcal 15-valent conjugate vaccine (PCV15) followed by a single dose of pneumococcal 23-valent polysaccharide vaccine (PPSV23) given at least 1 year after the dose of PCV15.[82,85] Individuals with chronic liver disease who previously received PPSV23 should receive one dose of PCV20 or PCV15, given at least 1 year after the dose of PPSV23 was administered, followed by a second dose of PPSV23 once they reach 65 years of age, ensuring there is a minimum of 5 years between the first and second dose of PPSV23.[82,85,86] For persons who previously received PCV13, the benefit of receiving PCV20 or PCV15 is not known; these individuals should receive a dose of PPSV23 at least one year following administration of PCV13.[82]
Table 5. Recommendations for Pneumococcal Immunization in Adults 19-64 Years of Age with Chronic Liver Disease
Prior Pneumococcal Vaccination Option A Option B None* (or pneumococcal vaccination history unknown) 1 dose PCV20 1 dose PCV15, followed ≥1 year with 1 dose of PPSV23 PPSV23 only 1 dose PCV20 (give ≥1 year after the last dose of PPSV23) 1 dose PCV15 (give ≥1 year after the last dose of PPSV23) PCV13 only 1 dose PCV20 (give ≥1 year after PCV13) 1 dose PPSV23 (give ≥1 year after PCV13); review pneumococcal immunization recommendations again when patient turns 65 years old PCV13 and PPSV23 No further pneumococcal immunization recommended at this time; review pneumococcal immunization recommendations again when patient turns 65 years old *Also applies to people who received PCV7 at any age and no other pneumococcal vaccines.
Source:- Advisory Committee on Immunization Practices (ACIP). Recommended Adult Immunization Schedule by Medical Condition and Other Indications, United States, 2025. [ACIP]
- Other Routine Vaccinations: Entry into care for the management of chronic HBV also presents an opportunity to ensure that the patient is up to date on other routine adult vaccinations, including yearly influenza vaccination COIVD-19 vaccinations, and a one-time tetanus diphtheria acellular pertussis (Tdap) vaccine followed by a tetanus diphtheria (Td) or Tdap booster every 10 years.[82]
Counseling Persons with Chronic HBV Following Diagnosis
Following the diagnosis of HBV, medical providers should counsel HBsAg-positive individuals on the natural history and key clinical aspects of HBV, expectations for follow-up care, the risk of HBV transmission to others, and ways to promote liver health, as outlined below.[13,87] For hepatitis B counseling resources in multiple languages, see the Washington State Department of Health Hep B Hub (Hepatitis B Educational Resource Hub).
- Natural history and key clinical aspects of HBV Infection
- Educate and counsel individuals on the long-term implications of chronic HBV infection, including the potential for developing cirrhosis and HCC.
- Advise persons with HBV to inform all medical providers of their HBsAg-positive status. This is of particular importance if the person requires chemotherapy or other immunosuppressive therapies for autoimmune or other immunologic diseases.
- Advise pregnant women and women of childbearing age that their newborns should receive both the hepatitis B vaccine and hepatitis B immune globulin (HBIG) at the time of birth.
- Expectations for follow-up care
- Counsel that HBV is a chronic illness that requires regular follow-up and monitoring at least every 6 months.
- Risk of HBV transmission to others
- Persons with HBV should verify that their household, sex, and needle-sharing partners have been screened and vaccinated for HBV.
- Advise persons with HBV to use barrier protection (e.g., condoms) during sexual intercourse to prevent transmission to susceptible partners.
- Advise persons with HBV to cover their cuts and clean up blood or bodily fluid spills with diluted bleach (ratio of 1:10 for bleach:water).
- Individuals with HBV should refrain from sharing items such as toothbrushes, razors, nail clippers, earrings, personal injection equipment, or other articles that may be contaminated with blood and pose a transmission risk to susceptible individuals.
- Advise people with HBV to not donate blood, plasma, tissue or semen.
- Counsel that HBV is not spread through kissing, hugging, coughing, sharing food or water, breastfeeding, or casual contact.
- General recommendations to promote liver health
- Counsel individuals with HBV to avoid alcohol.
- Advise persons with HBV to maintain a healthy body weight and control their blood sugars and cholesterol to prevent the development of nonalcoholic steatohepatitis.
- Recommend persons with HBV receive hepatitis A vaccine if they are not immune to HAV.
- For guidance regarding over-the-counter medications, complementary and alternative therapies, and supplements, see the lesson Counseling Persons with Chronic HCV Infection on the Hepatitis C Online website.
Summary Points
- After confirming chronic infection with HBV, the medical provider should perform a detailed history aimed at identifying risk factors for acquiring HBV, evaluating for significant medical comorbidities, and understanding any prior evaluation and treatment for HBV.
- A complete physical examination should be performed, focusing on stigmata of chronic liver disease, including ascites, caput medusae, gynecomastia, jaundice, palmar erythema, spider angiomata, and Terry’s nails.
- The initial laboratory evaluation should include a complete blood count, chemistry panel with creatinine and hepatic function testing, hepatitis B serologic and DNA testing, and testing for HIV and HCV.
- Clinicians should be familiar with the most important nonviral causes of hepatic inflammation, but an exhaustive screening laboratory work-up for other causes of liver disease is usually not required because of the high cost and low yield.
- People with chronic HBV should receive routinely recommended adult immunizations, as well as hepatitis A and pneumococcal vaccines.
- Persons with HBV should receive counseling on the natural history and key clinical aspects of HBV Infection, as well as general recommendations on how to promote liver health.
Citations
- 1.Schillie S, Vellozzi C, Reingold A, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.[PubMed Abstract] -
- 2.Mushahwar IK, Dienstag JL, Polesky HF, McGrath LC, Decker RH, Overby LR. Interpretation of various serological profiles of hepatitis B virus infection. Am J Clin Pathol. 1981;76:773-7.[PubMed Abstract] -
- 3.Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053-63.[PubMed Abstract] -
- 4.Fontana RJ. Evaluation of the patient with chronic hepatitis B. Clin Liver Dis (Hoboken). 2013;2:1-4.[PubMed Abstract] -
- 5.Rotman Y, Brown TA, Hoofnagle JH. Evaluation of the patient with hepatitis B. Hepatology. 2009;49:S22-7.[PubMed Abstract] -
- 6.Centers for Disease Control and Prevention (CDC). 2022 Viral Hepatitis Surveillance Report—Hepatitis B. Published April 2024.[CDC] -
- 7.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-55.[PubMed Abstract] -
- 8.World Health Organization. Global hepatitis report, 2017. April, 2017:1-68.[WHO] -
- 9.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323-31.[PubMed Abstract] -
- 10.Iida-Ueno A, Enomoto M, Tamori A, Kawada N. Hepatitis B virus infection and alcohol consumption. World J Gastroenterol. 2017;23:2651-9.[PubMed Abstract] -
- 11.Ikeda K, Saitoh S, Suzuki Y, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930-8.[PubMed Abstract] -
- 12.Lin CW, Lin CC, Mo LR, et al. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J Hepatol. 2013;58:730-5.[PubMed Abstract] -
- 13.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-99.[PubMed Abstract] -
- 14.National Institute on Alcohol Abuse and Alcoholism. Rethinking drinking: alcohol and your health. What counts as a Drink?[NIAA] -
- 15.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905-7.[PubMed Abstract] -
- 16.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-95.[PubMed Abstract] -
- 17.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791-804.[PubMed Abstract] -
- 18.Tang AS, Thornton K, and HBV Primary Care Workgroup. Hepatitis B Management: Guidance for the Primary Care Provider. February 25, 2020.
- 19.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-76.[PubMed Abstract] -
- 20.European Association For The Study Of The Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-98.[PubMed Abstract] -
- 21.Lai CL, Dienstag J, Schiff E, et al. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36:687-96.[PubMed Abstract] -
- 22.Han SH. Extrahepatic manifestations of chronic hepatitis B. Clin Liver Dis. 2004;8:403-18.[PubMed Abstract] -
- 23.Guillevin L, Lhote F, Cohen P, et al. Polyarteritis nodosa related to hepatitis B virus. A prospective study with long-term observation of 41 patients. Medicine (Baltimore). 1995;74:238-53.[PubMed Abstract] -
- 24.Lai KN, Lai FM, Chan KW, Chow CB, Tong KL, Vallance-Owen J. The clinico-pathologic features of hepatitis B virus-associated glomerulonephritis. Q J Med. 1987;63:323-33.[PubMed Abstract] -
- 25.Johnson RJ, Couser WG. Hepatitis B infection and renal disease: clinical, immunopathogenetic and therapeutic considerations. Kidney Int. 1990;37:663-76.[PubMed Abstract] -
- 26.Mazzaro C, Adinolfi LE, Pozzato G, et al. Extrahepatic Manifestations of Chronic HBV Infection and the Role of Antiviral Therapy. J Clin Med. 2022;11:1:6247.[PubMed Abstract] -
- 27.Win NN, Kanda T, Ogawa M, et al. Superinfection of hepatitis A virus in hepatocytes infected with hepatitis B virus. Int J Med Sci. 2019;16:1366-70.[PubMed Abstract] -
- 28.Jamma S, Hussain G, Lau DT. Current Concepts of HBV/HCV Coinfection: Coexistence, but Not Necessarily in Harmony. Curr Hepat Rep. 2010;9:260-9.[PubMed Abstract] -
- 29.Shah PA, Choudhry S, Reyes KJC, Lau DTY. An update on the management of chronic hepatitis D. Gastroenterol Rep (Oxf). 2019;7:396-402.[PubMed Abstract] -
- 30.Stockdale AJ, Kreuels B, Henrion MYR, et al. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J Hepatol. 2020;73:523-32.[PubMed Abstract] -
- 31.Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS. 2017;31:2035-2052.[PubMed Abstract] -
- 32.McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir - effects on HIV-1 replication and resistance. N Engl J Med. 2007;356:2614-21.[PubMed Abstract] -
- 33.Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Hepatitis B virus infection. Last Updated: December 16, 2024.[HIV.gov] -
- 34.Nelson DR, Teckman J, Di Bisceglie AM, Brenner DA. Diagnosis and management of patients with α1-antitrypsin (A1AT) deficiency. Clin Gastroenterol Hepatol. 2012;10:575-80.[PubMed Abstract] -
- 35.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54-66.[PubMed Abstract] -
- 36.Czaja AJ. Review article: next-generation transformative advances in the pathogenesis and management of autoimmune hepatitis. Aliment Pharmacol Ther. 2017;46:920-37.[PubMed Abstract] -
- 37.Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:328-43.[PubMed Abstract] -
- 38.Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-56.[PubMed Abstract] -
- 39.Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063-72.[PubMed Abstract] -
- 40.de Bruyn G, Graviss EA. A systematic review of the diagnostic accuracy of physical examination for the detection of cirrhosis. BMC Med Inform Decis Mak. 2001;1:6.[PubMed Abstract] -
- 41.Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74:756-62.[PubMed Abstract] -
- 42.Satapathy SK, Bernstein D. Dermatologic disorders and the liver. Clin Liver Dis. 2011;15:165-82.[PubMed Abstract] -
- 43.Udell JA, Wang CS, Tinmouth J, et al. Does this patient with liver disease have cirrhosis? JAMA. 2012;307:832-42.[PubMed Abstract] -
- 44.Cattau EL Jr, Benjamin SB, Knuff TE, Castell DO. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-6.[PubMed Abstract] -
- 45.Kim Sh, Keum B, Kim E, Jeen Y, Chun H. Hepatobiliary and pancreatic: Caput medusae. J Gastroenterol Hepatol. 2014;29:1952.[PubMed Abstract] -
- 46.Yang PM, Chen DS. Images in clinical medicine. Caput medusae. N Engl J Med. 2005;353:e19.[PubMed Abstract] -
- 47.Braunstein GD. Clinical practice. Gynecomastia. N Engl J Med. 2007;357:1229-37.[PubMed Abstract] -
- 48.Dickson G. Gynecomastia. Am Fam Physician. 2012;85:716-22.[PubMed Abstract] -
- 49.Ruiz MA, Saab S, Rickman LS. The clinical detection of scleral icterus: observations of multiple examiners. Mil Med. 1997;162:560-3.[PubMed Abstract] -
- 50.Serrao R, Zirwas M, English JC. Palmar erythema. Am J Clin Dermatol. 2007;8:347-56.[PubMed Abstract] -
- 51.Khasnis A, Gokula RM. Spider nevus. J Postgrad Med. 2002;48:307-9.[PubMed Abstract] -
- 52.Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of vascular endothelial growth factor and basic fibroblast growth factor. World J Gastroenterol. 2003;9:2832-5.[PubMed Abstract] -
- 53.Holzberg M, Walker HK. Terry's nails: revised definition and new correlations. Lancet. 1984;1:896-9.[PubMed Abstract] -
- 54.Pitukweerakul S, Pilla S. Terry's Nails and Lindsay's Nails: Two Nail Abnormalities in Chronic Systemic Diseases. J Gen Intern Med. 2016;31:970.[PubMed Abstract] -
- 55.McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17:646-9.[PubMed Abstract] -
- 56.Singal AG, Llovet JM, Yarchoan M, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922-65.[PubMed Abstract] -
- 57.Batts KP. Iron overload syndromes and the liver. Mod Pathol. 2007;20 Suppl 1:S31-9.[PubMed Abstract] -
- 58.Tome S, Lucey MR. Review article: current management of alcoholic liver disease. Aliment Pharmacol Ther. 2004;19:707-14.[PubMed Abstract] -
- 59.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. 2018;113:175-194.[PubMed Abstract] -
- 60.Cohen JA, Kaplan MM. The SGOT/SGPT ratio--an indicator of alcoholic liver disease. Dig Dis Sci. 1979;24:835-8.[PubMed Abstract] -
- 61.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-69.[PubMed Abstract] -
- 62.Dugum M, McCullough A. Diagnosis and Management of Alcoholic Liver Disease. J Clin Transl Hepatol. 2015;3:109-16.[PubMed Abstract] -
- 63.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20.[PubMed Abstract] -
- 64.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.[PubMed Abstract] -
- 65.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-609.[PubMed Abstract] -
- 66.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-57.[PubMed Abstract] -
- 67.Brandhagen DJ, Fairbanks VF, Baldus W. Recognition and management of hereditary hemochromatosis. Am Fam Physician. 2002;65:853-60.[PubMed Abstract] -
- 68.Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med. 2012;366:348-59.[PubMed Abstract] -
- 69.Kowdley KV. Iron Overload in Patients With Chronic Liver Disease. Gastroenterol Hepatol (N Y). 2016;12:695-8.[PubMed Abstract] -
- 70.Qaseem A, Aronson M, Fitterman N, Snow V, Weiss KB, Owens DK. Screening for hereditary hemochromatosis: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005;143:517-21.[PubMed Abstract] -
- 71.Schmitt B, Golub RM, Green R. Screening primary care patients for hereditary hemochromatosis with transferrin saturation and serum ferritin level: systematic review for the American College of Physicians. Ann Intern Med. 2005;143:522-36.[PubMed Abstract] -
- 72.Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis--Update 2015. J Hepatol. 2015;62:S100-11.[PubMed Abstract] -
- 73.Mieli-Vergani G, Vergani D, Czaja AJ, et al. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4:18017.[PubMed Abstract] -
- 74.Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479-97.[PubMed Abstract] -
- 75.Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193-213.[PubMed Abstract] -
- 76.Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-76.[PubMed Abstract] -
- 77.Mack CL, Adams D, Assis DN, et al. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671-722.[PubMed Abstract] -
- 78.Marciniak SJ, Lomas DA. Alpha1-antitrypsin deficiency and autophagy. N Engl J Med. 2010;363:1863-4.[PubMed Abstract] -
- 79.Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet. 2005;365:2225-36.[PubMed Abstract] -
- 80.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293-1302.e4.[PubMed Abstract] -
- 81.Theise ND. Liver biopsy assessment in chronic viral hepatitis: a personal, practical approach. Mod Pathol. 2007;20 Suppl 1:S3-14.[PubMed Abstract] -
- 82.Advisory Committee on Immunization Practices (ACIP). Adult Immunization Schedule: Recommended Immunization Schedule for Ages 19 Years or Older, United States, 2025.[ACIP] -
- 83.Nelson NP, Weng MK, Hofmeister MG, et al. Prevention of Hepatitis A Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Recomm Rep. 2020;69:1-38.[PubMed Abstract] -
- 84.Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55:1-23.[PubMed Abstract] -
- 85.Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-Valent Pneumococcal Conjugate Vaccine and 20-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Updated Recommendations of the Advisory Committee on Immunization Practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109-17.[PubMed Abstract] -
- 86.Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged ≥65 Years: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-75.[PubMed Abstract] -
- 87.Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1-20.[PubMed Abstract] -
Additional References
- Advisory Committee on Immunization Practices (ACIP). Recommended Adult Immunization Schedule by Medical Condition and Other Indications, United States, 2025.[ACIP] -
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-9.[PubMed Abstract] -
- Centers for Disease Control and Prevention. Vaccine information for adults: liver disease and adult vaccination.[CDC] -
- Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717-1730.[PubMed Abstract] -
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-50.[PubMed Abstract] -
- Pappachan JM, Babu S, Krishnan B, Ravindran NC. Non-alcoholic Fatty Liver Disease: A Clinical Update. J Clin Transl Hepatol. 2017;5:384-93.[PubMed Abstract] -
- Pascale A, Pais R, Ratziu V. An overview of nonalcoholic steatohepatitis: past, present and future directions. J Gastrointestin Liver Dis. 2010;19:415-23.[PubMed Abstract] -
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44-100.[PubMed Abstract] -
Figures
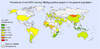 Figure 1. Global Prevalence of anti-HDV Among HBsAg-Positive PeopleSource: Stockdale AJ, Kreuels B, Henrion MYR, et al. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J Hepatol. 2020;73:523-32.
Figure 1. Global Prevalence of anti-HDV Among HBsAg-Positive PeopleSource: Stockdale AJ, Kreuels B, Henrion MYR, et al. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J Hepatol. 2020;73:523-32. Figure 2. Body Mass Index (BMI) FormulaBody Mass Index (BMI) represents a number calculated based on a person's weight and height and it provides a good rough estimate of a person's body fat. The BMI may overestimate body fat in athletes and underestimate body fat in older persons, or individuals who have lost significant muscle.Source: National Heart Lung and Blood Institute
Figure 2. Body Mass Index (BMI) FormulaBody Mass Index (BMI) represents a number calculated based on a person's weight and height and it provides a good rough estimate of a person's body fat. The BMI may overestimate body fat in athletes and underestimate body fat in older persons, or individuals who have lost significant muscle.Source: National Heart Lung and Blood Institute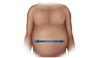 Figure 3 (Image Series). Physical Examination Findings in Patients with CirrhosisThe presence of bulging flanks suggests a possible diagnosis of ascites; this should be confirmed with a shifting dullness test.Illustration from Cognition Studio, Inc.
Figure 3 (Image Series). Physical Examination Findings in Patients with CirrhosisThe presence of bulging flanks suggests a possible diagnosis of ascites; this should be confirmed with a shifting dullness test.Illustration from Cognition Studio, Inc.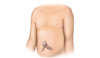 Figure 3B. Caput MedusaCaput medusa results from portal hypertension and is manifested as distended abdominal veins radiating around the umbilicus.Illustration from Cognition Studio, Inc.
Figure 3B. Caput MedusaCaput medusa results from portal hypertension and is manifested as distended abdominal veins radiating around the umbilicus.Illustration from Cognition Studio, Inc.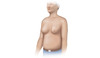 Figure 3C. GynecomastiaIn men with cirrhosis, benign enlargement of the breasts may occur and manifest as gynecomastia.Illustration from Cognition Studio, Inc.
Figure 3C. GynecomastiaIn men with cirrhosis, benign enlargement of the breasts may occur and manifest as gynecomastia.Illustration from Cognition Studio, Inc. Figure 3D. JaundiceThis illustration shows yellow discoloration of the sclera that results from excess deposition of biliary pigments.Illustration from Cognition Studio, Inc.
Figure 3D. JaundiceThis illustration shows yellow discoloration of the sclera that results from excess deposition of biliary pigments.Illustration from Cognition Studio, Inc.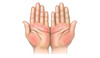 Figure 3E. Palmar ErythemaThe palmar erythema is most prominent in the thenar and hypothenar eminence, with sparing of the central palm.Illustration from Cognition Studio, Inc.
Figure 3E. Palmar ErythemaThe palmar erythema is most prominent in the thenar and hypothenar eminence, with sparing of the central palm.Illustration from Cognition Studio, Inc.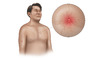 Figure 3F. Spider AngiomataThe enlarged cutaneous blood vessels that resemble a spider.Illustration from Cognition Studio, Inc.
Figure 3F. Spider AngiomataThe enlarged cutaneous blood vessels that resemble a spider.Illustration from Cognition Studio, Inc. Figure 3G. Terry's NailsNote the white-silver discoloration of the proximal nail bed and the pink band on the distal portion of the nail bed.Illustration from Cognition Studio, Inc.
Figure 3G. Terry's NailsNote the white-silver discoloration of the proximal nail bed and the pink band on the distal portion of the nail bed.Illustration from Cognition Studio, Inc.Tables
Table 1. Global Prevalence of Chronic HBV Infection, by Country
Prevalence Category Country High
(≥8%)Angola, Cabo Verde, Central African Republic, Chad, Eswatini, Ghana, Guinea, Guinea-Bissau, Kiribati, Lesotho, Liberia, Mali, Mauritania, Niger, Nigeria, Philippines, Sao Tome and Principe, Sierra Leone, Solomon Islands, Taiwan, Timor-Leste, Togo, Tonga, Turkmenistan, Tuvalu, and Zimbabwe. Intermediate
(5.0-7.9%)Albania, Benin, Burkina Faso, Cameroon, China, Côte d’Ivoire, Democratic People’s Republic of Korea, Djibouti, Eritrea, Ethiopia, Federated States of Micronesia, Gabon, Indonesia, Kyrgyzstan, Moldova, Mongolia, Mozambique, Myanmar, Papua New Guinea, Senegal, Somalia, South Sudan, Syria, Tajikistan, Uzbekistan, Vanuatu, and Vietnam. Low Intermediate
(2.0-4.9%)Afghanistan, Azerbaijan, Bangladesh, Belarus, Bosnia and Herzegovina, Bulgaria, Burundi, Cambodia, Comoros, Congo, Democratic Republic of Congo, Gambia, Georgia, Guyana, Haiti, Hong Kong, India, Iraq, Jamaica, Jordan, Kazakhstan, South Korea, Laos, Madagascar, Malawi, Malaysia, Marshall Islands, Oman, Pakistan, Romania, Rwanda, Samoa, Singapore, South Africa, Sri Lanka, Sudan, Tanzania, Thailand, Trinidad and Tobago, Tunisia, Turkey, Uganda, Yemen, and Zambia. Low
(≤1.9%)Algeria, Argentina, Armenia, Australia, Austria, Bahrain, Belgium, Belize, Bhutan, Bolivia, Brazil, Canada, Chile, Colombia, Costa Rica, Croatia, Cuba, Czechia, Denmark, Dominican Republic, Ecuador, Egypt, El Salvador, Estonia, Fiji, Finland, France, Germany, Greece, Guatemala, Honduras, Hungary, Iran, Ireland, Israel, Italy, Japan, Kenya, Kosovo, Kuwait, Lebanon, Libya, Mexico, Morocco, Nepal, Netherlands, New Zealand, Nicaragua, Norway, Palestine, Panama, Paraguay, Peru, Poland, Portugal, Qatar, Russia, Saudi Arabia, Slovakia, Slovenia, Spain, Suriname, Sweden, Switzerland, Ukraine, United Arab Emirates, United Kingdom, United States, and Venezuela. Unknown prevalence (data not available)
American Samoa, Andorra, Anguilla, Antigua and Barbuda, Aruba, Bahamas, Barbados, Bermuda, Bonaire Sint Eustatius and Saba, Botswana, British Virgin Islands, Brunei, Cayman Islands, Cook Islands, Curaçao, Cyprus, Dominica, Equatorial Guinea, Falkland Islands, Faroe Islands, French Guiana, French Polynesia, Gibraltar, Greenland, Grenada, Guadeloupe, Guam, Holy See, Iceland, Isle of Man, Latvia, Liechtenstein, Lithuania, Luxembourg, Macao, Macedonia, Maldives, Malta, Martinique, Mauritius, Mayotte, Monaco, Montenegro, Montserrat, Namibia, Nauru, New Caledonia, Niue, Northern Mariana Islands, Palau, Puerto Rico, Réunion, Saint Barthélemy, Saint Helena, Saint Kitts and Nevis, Saint Lucia, Saint Martin, Saint Pierre and Miquelon, Saint Vincent and the Grenadines, San Marino, Serbia, Seychelles, Sint Maarten, Tokelau, Turks and Caicos Islands, U.S. Virgin Islands, Uruguay, Wallis and Futuna, and Western Sahara. NOTE: This table is based on data from the Centers for Disease Control and Prevention (CDC) Source:- Conners EE, Panagiotakopoulos L, Hofmeister MG, et al. Screening and Testing for Hepatitis B Virus Infection: CDC Recommendations - United States, 2023. MMWR Recomm Rep. 2023;72:1-25. [PubMed Abstract]
Table 2. Key Characteristics of Oral Antiviral Agents Used to Treat HBV and/or HIV*
Hepatitis B Virus HIV Medication Potency Against HBV Barrier to HBV Resistance Potency Against HIV Barrier to HIV Resistance Adefovir Low Moderate Weak Low Entecavir High High Weak Low Lamivudine Moderate Low Medium Low Tenofovir alafenamide High High Medium Medium Tenofovir DF High High Medium Medium *Telbivudine is not included as it is no longer manufactured in the United States
Table 3. Diagnostic Accuracy of the Physical Examination for Detecting Cirrhosis
Finding Sensitivity Specificity Positive LR (95% CI) Terry nails 0.43-0.44 0.97-0.98 16-22 Gynecomastia 0.18-0.58 0.97-0.98 5.8-35 Distended abdominal veinsa 0.31 0.98 11 (2.7-44) Encephalopathyb 0.16 0.98 10 (1.5-77) Decreased body haira 0.36 0.97 9.0 (6.4-13) Ascitesb 0.35 0.95 7.2 (2.9-12) Facial telangiectasia 0.73-0.82 0.88-0.92 5.9-10 Testicular atrophy 0.18 0.97 5,8 (2.4-14) Palmar erythemab 0.46 0.91 5.0 (0.80-9.1) Spider nevia 0.46 0.89 4.3 (2.4-6.2) Jaundicea 0.28 0.93 3.8 (2.0-7.2) Splenomegalyb 0.34 0.90 3.5 (1.8-5.2) Firm livera 0.73 0.81 3.3 (2.3-4.7) Peripheral edemaa 0.37 0.90 3.0 (1.9-4.8) Hepatomegalyb 0,74 0.69 2.4 (1.2-3.6) Obesitya 0.64 0.52 1.3 (1.1-1.6) *Abbreviations: LR = likelihood ratio; CI = confidence interval
a = Univariate random-effects summary measures because data did not converge on a bivariate solution
b = Bivariate random-effects summary measuresSource:- Udell JA, Wang CS, Tinmouth J, et al. Does this patient with liver disease have cirrhosis? JAMA. 2012;307:832-42. [PubMed Abstract]
Table 4. Recommended Hepatitis A Immunization for Adults
Vaccine Dosage Dosing and Route Hepatitis A Vaccines
Havrix 1440 EL.U 2-Dose Schedule: 1 mL given IM at 0 and 6-12 months Vaqta 50 U 2-Dose Schedule: 1 mL given IM at 0 and 6-18 months Combined Hepatitis A and B Vaccine
Twinrix HAV: 720 EL.U
plus
HBsAg: 20 mcgStandard 3-dose series: 1 mL given IM at 0, 1, and 6 months
Accelerated 4-dose series: 1 mL given IM on days 0, 7, and 21-30, followed by a booster dose at month 12Abbreviations: IM = intramuscular; HAV = hepatitis A virus; HBsAg = hepatitis B surface antigen
Table 5. Recommendations for Pneumococcal Immunization in Adults 19-64 Years of Age with Chronic Liver Disease
Prior Pneumococcal Vaccination Option A Option B None* (or pneumococcal vaccination history unknown) 1 dose PCV20 1 dose PCV15, followed ≥1 year with 1 dose of PPSV23 PPSV23 only 1 dose PCV20 (give ≥1 year after the last dose of PPSV23) 1 dose PCV15 (give ≥1 year after the last dose of PPSV23) PCV13 only 1 dose PCV20 (give ≥1 year after PCV13) 1 dose PPSV23 (give ≥1 year after PCV13); review pneumococcal immunization recommendations again when patient turns 65 years old PCV13 and PPSV23 No further pneumococcal immunization recommended at this time; review pneumococcal immunization recommendations again when patient turns 65 years old *Also applies to people who received PCV7 at any age and no other pneumococcal vaccines.
Source:- Advisory Committee on Immunization Practices (ACIP). Recommended Adult Immunization Schedule by Medical Condition and Other Indications, United States, 2025. [ACIP]
Share by e-mail
Check
-On-
Learning
QuestionsThe Check-on-Learning Questions are short and topic related. They are meant to help you stay on track throughout each lesson and check your understanding of key concepts.You must be signed in to customize your interaction with these questions.
- HBV Medications
- Self-Study Modules CNE/CME
- HBV Epidemiology
- HBV Screening, Testing, and Diagnosis
- HBV Immunizations
- Initial Evaluation of Persons with Chronic Hepatitis B
- When to Initiate HBV Treatment
- Choosing an Initial HBV Treatment Regimen
- Monitoring Persons On and Off HBV Therapy
- Preventing HBV Perinatal Transmission
- Screening for Hepatocellular Carcinoma
- Occupational HBV Postexposure Prophylaxis
- Hepatitis B Reactivation in the Setting of Immunosuppression
Quick Reference
- HBV Epidemiology
- HBV Screening, Testing, and Diagnosis
- HBV Immunizations
- Initial Evaluation of Persons with Chronic Hepatitis B
- When to Initiate HBV Treatment
- Choosing an Initial HBV Treatment Regimen
- Monitoring Persons On and Off HBV Therapy
- Preventing HBV Perinatal Transmission
- Screening for Hepatocellular Carcinoma
- Occupational HBV Postexposure Prophylaxis
- Hepatitis B Reactivation in the Setting of Immunosuppression
- Clinical ChallengesHepatitis B Primary Care Guidance Master Bibliography
Contributors - Tools & Calculators
Since you've received 80% or better on this quiz, you may claim continuing education credit.
You seem to have a popup blocker enabled. If you want to skip this dialog please Always allow popup windows for the online course.