Peginterferon alfa-2a (Pegasys)
Boxed Warning
WARNING: RISK OF SERIOUS DISORDERS
Risk of Serious Disorders
Alpha interferons, including PEGASYS (peginterferon alfa-2a), may cause or aggravate fatal or life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Patients should be monitored closely with periodic clinical and laboratory evaluations. Therapy should be withdrawn in patients with persistently severe or worsening signs or symptoms of these conditions. In many, but not all cases, these disorders resolve after stopping PEGASYS therapy [see Warnings and Precautions (5.2, 5.5, 5.8, 5.11, 5.14, 5.16), Adverse Reactions (6.1) and Nonclinical Toxicology (13.1)].
1. Indications and Usage
1.1 Chronic Hepatitis C (CHC)
Adult Patients: PEGASYS, as part of a combination regimen with other hepatitis C virus (HCV) antiviral drugs, is indicated for the treatment of adults with CHC and compensated liver disease. For information about the safe and effective use of other HCV antiviral drugs to be used in combination with PEGASYS, refer to their prescribing information. PEGASYS monotherapy is only indicated for the treatment of patients with CHC and compensated liver disease if there are contraindications or significant intolerance to other HCV antiviral drugs.
Pediatric Patients: PEGASYS in combination with ribavirin is indicated for the treatment of pediatric patients 5 years of age and older with CHC and compensated liver disease.
Limitations of Use:
- PEGASYS alone or in combination with ribavirin without additional HCV antiviral drugs is not recommended for treatment of patients with CHC who previously failed therapy with an interferon-alfa.
- PEGASYS is not recommended for treatment of patients with CHC who have had solid organ transplantation [see Use in Specific Populations (8.8)].
2. Dosage and Administration
2.1 Dosage Overview
Administer PEGASYS by subcutaneous injection once weekly in the abdomen or thigh for the treatment of:
- Adult patients with CHC without or with HIV coinfection [see Dosage and Administration (2.2)];
- Pediatric patients with CHC [see Dosage and Administration (2.3)];
- Adult patients with CHB [see Dosage and Administration (2.4)]; and
- Pediatric patients with CHB [see Dosage and Administration (2.5)].
For treatment of CHC, use PEGASYS in combination with other HCV antiviral drugs. For information about the recommended dosage and administration and the safe and effective use of these other HCV antiviral drugs, refer to their prescribing information. PEGASYS monotherapy is only indicated in the treatment of CHC if there are contraindications to or significant intolerance to other HCV antiviral drugs.
For dosage modifications in patients with CHC or CHB:
- Due to neutropenia, thrombocytopenia, ALT elevation, and depression [see Dosage and Administration (2.6)].
- In patients with severe renal impairment (creatinine clearance less than 30 mL/minute) and in patients with creatinine clearance between 30 and 50 mL/minute [see Dosage and Administration (2.6)].
For important administration instructions for all the PEGASYS injection presentations (i.e., vial and prefilled syringe) [see Dosage and Administration (2.6)].
2.2 Recommended Dosage for Adults with CHC
Dosage in Adults with CHC without HIV Coinfection
Table 1 displays the recommended dosage and duration of PEGASYS and other HCV antiviral drugs in adults with CHC (without HIV coinfection) based on HCV genotype.
For treatment of HCV genotype 1 with PEGASYS in combination with ribavirin or alone, discontinuation of treatment is recommended if at least a 2 log10 reduction from baseline in HCV RNA has not been demonstrated by 12 weeks of therapy or if undetectable HCV RNA has not been achieved after 24 weeks of therapy [see Clinical Studies (14)]. Refer to the prescribing information for specific HCV antiviral drugs used in combination with PEGASYS for information on stopping therapy based on treatment response.
Immediately discontinue PEGASYS for hepatic decompensation (Child-Pugh score greater than 6 [class B and C]).
| Hepatitis C Virus Genotype | PEGASYS Dosage | PEGASYS Duration |
|---|---|---|
| Genotypes 1, 4† | 180 mcg subcutaneous injection in thigh or abdomen once weekly | Refer to the prescribing information of HCV antiviral drugs. |
| Genotypes 2, 3‡ | ||
| Genotypes 5, 6 | There are insufficient data for dosage recommendations | |
| ||
If PEGASYS monotherapy is used for treatment of CHC, the recommended PEGASYS dosage is 180 mcg via subcutaneous injection in thigh or abdomen once weekly for 48 weeks.
Dosage in Adults with CHC with HIV Coinfection
The recommended PEGASYS dosage in adults with CHC and HIV coinfection is 180 mcg subcutaneously once weekly in the thigh or abdomen. If PEGASYS is used in combination with other antiviral drugs, refer to the prescribing information of the other HCV antiviral drugs for the recommended dosage of the other HCV antiviral drugs and duration of the entire treatment regimen (including PEGASYS). If PEGASYS and ribavirin are used without other HCV antiviral drugs, the recommended duration of therapy is 48 weeks (regardless of HCV genotype).
2.3 Recommended Dosage for Pediatric Patients with CHC
PEGASYS is administered as 180 mcg/1.73 m2 × BSA subcutaneously once weekly, to a maximum dose of 180 mcg, and should be given in combination with ribavirin. The recommended treatment duration for pediatric patients with HCV genotype 2 or 3 is 24 weeks and for other HCV genotypes is 48 weeks. Patients who initiate treatment prior to their 18th birthday should maintain the recommended pediatric dosage (not the adult dosage) through the completion of therapy. Refer to the prescribing information of ribavirin for the recommended dosage and duration.
2.4 Recommended Dosage for Adults with CHB
The recommended PEGASYS dosage in adults with CHB is 180 mcg subcutaneously once weekly in the thigh or abdomen for 48 weeks.
2.5 Recommended Dosage for Pediatrics Patients with CHB
The recommended PEGASYS dosage in pediatric patients for HBeAg-positive CHB is 180 mcg/1.73 m2 × BSA subcutaneously once weekly to a maximum dose of 180 mcg. The recommended duration of therapy is 48 weeks.
Maintain the recommended pediatric dosage through the entire duration of therapy in patients who turn 18 years of age during therapy.
2.6 Dosage Modifications
PEGASYS Dosage Modifications Due to Adverse Reactions, Neutropenia or Thrombocytopenia in Adults
Table 2 displays the recommended PEGASYS dosage modifications due to adverse reactions, or due to neutropenia, or thrombocytopenia in adults. Following improvement of the adverse reaction, neutropenia or thrombocytopenia, consider re-escalation of the dosage back to the previous dosage [see Warnings and Precautions (5) and Adverse Reactions (6)].
| Laboratory Values | Recommended PEGASYS Dosage |
|---|---|
| ANC = absolute neutrophil count | |
| Neutropenia | |
| ANC 500 to less than 750 cells/mm3 | Reduce to 135 mcg subcutaneously once weekly |
| ANC less than 500 cells/mm3 | Discontinue treatment until ANC values return to more than 1000 cells/mm3. Reinstitute at 90 mcg subcutaneously once weekly and monitor ANC. |
| Thrombocytopenia | |
| Platelet 25,000 to less than 50,000 cells/mm3 | Reduce to 90 mcg subcutaneously once weekly |
| Platelet less than 25,000 cells/mm3 | Discontinue treatment |
PEGASYS Dosage Modifications Due to ALT Elevation in Adults
If ALT increases are progressive despite dose reduction or accompanied by increased bilirubin or evidence of hepatic decompensation, therapy should be immediately discontinued. In CHC patients with progressive ALT increases above baseline values, the dosage of PEGASYS should be reduced to 135 mcg and more frequent monitoring of liver function should be performed. After PEGASYS dosage reduction or withholding, therapy can be resumed after ALT flares subside.
In CHB patients with elevations in ALT (greater than 5 × ULN), more frequent monitoring of liver function should be performed and consideration should be given to either reducing the dosage of PEGASYS to 135 mcg or temporarily discontinuing treatment. After PEGASYS dosage reduction or withholding, therapy can be resumed after ALT flares subside.
In adult patients with persistent, severe (ALT greater than 10 times above the upper limit of normal) hepatitis B flares, consideration should be given to discontinuation of treatment.
PEGASYS Dosage Modifications Due to Depression in Adults and in Pediatric Patients
Table 3 displays the recommended PEGASYS dosage modifications in adult and pediatric patients who develop interferon-related depression or whose underlying depression worsens. Table 3 also includes recommended frequency of psychiatric visits.
| Depression Severity | Initial Depression Management (4-8 weeks) | Depression Management After 8 Weeks | |||
|---|---|---|---|---|---|
| Dosage Modification | Visit Schedule | Depression Severity Remains Stable | Depression Severity Improves | Depression Severity Worsens | |
| Mild | No change | Evaluate once weekly by visit and/or phone | Continue weekly visit schedule | Resume normal visit schedule | Consider psychiatric consultation. Discontinue PEGASYS or reduce dosage to 135 mcg in adults (135 mcg/1.73 m2 × BSA for pediatric patients) or 90 mcg once weekly for adults (90 mcg/1.73 m2 × BSA for pediatric patients) |
| Moderate | Decrease PEGASYS dosage to 135 mcg in adults (135 mcg/1.73 m2 × BSA for pediatric patients) or 90 mcg in adults (90 mcg/1.73 m2 × BSA for pediatric patients) once weekly | Evaluate once weekly (office visit at least every other week) | Consider psychiatric consultation. Continue reduced dosing | If symptoms improve and are stable for 4 weeks, may resume normal visit schedule. Continue reduced dosage or return to normal dosage | Obtain immediate psychiatric consultation Discontinue PEGASYS permanently. |
| Severe | Discontinue PEGASYS permanently | Obtain immediate psychiatric consultation | Psychiatric therapy necessary | ||
PEGASYS Dosage Modifications Due to Adverse Reactions or Laboratory Abnormalities in Pediatric Patients
Table 4 displays the recommended PEGASYS dosage modifications due to adverse reactions, neutropenia, thrombocytopenia, or elevated ALT in pediatric patients.
| Laboratory Abnormality | Recommended PEGASYS Dosage Modification | ||
|---|---|---|---|
| CHC | CHB | ||
| ANC = absolute neutrophil count | |||
| Neutropenia | ANC 750-999 cells/mm3 | Week 1-2: immediate modification to 135 mcg/1.73 m2 × BSA Week 3-48: no modification. | No dosage modification. |
| ANC 500-749 cells/mm3 | Week 1-2:
Week 3-48: immediate modification to 135 mcg/1.73 m2 × BSA. | Immediate modification to 135 mcg/1.73 m2 × BSA. | |
| ANC 250-499 cells/mm3 | Week 1-2: Delay or hold dosage until ANC is more than 750 cells/mm3 then resume dose at 90 mcg/1.73 m2 × BSA; Week 3-48: Delay or hold dosage until ANC is more than 750 cells/mm3 then resume dosage at 135 mcg/1.73 m2 × BSA. | Interrupt dosing until ANC is more than or equal to 1000 cells/mm3, then resume dose with 90 mcg/1.73 m2 × BSA and monitor. | |
| ANC less than 250 cells/mm3 (or febrile neutropenia): | Discontinue treatment. | Discontinue treatment. | |
| Thrombocytopenia | Platelet 25,000 to less than 50,000 cells/mm3 | Reduce dosage to 90 mcg/1.73 m2 × BSA. | Reduce dosage to 90 mcg/1.73 m2 × BSA. |
| Platelet less than 25,000 cells/mm3 | Discontinue treatment. | Discontinue treatment. | |
| Increased alanine transaminase (ALT) | For persistent or increasing elevations more than or equal to 5 but less than 10 × ULN |
|
|
| For persistent ALT values more than or equal to 10 × ULN | Discontinue treatment. | Discontinue treatment. | |
PEGASYS Dosage Modifications for Adults with Renal Impairment
Prior to administering PEGASYS, evaluate renal function. Table 5 displays the recommended dosage modifications for adults with creatinine clearance less than 30 mL/minute, including patients with end-stage renal disease requiring hemodialysis [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)]. Refer to the respective prescribing information of other HCV antiviral drugs regarding use in patients with renal impairment.
| Creatinine Clearance | Recommended PEGASYS Dosage |
|---|---|
| 30 to 50 mL/minute | 180 mcg once weekly |
| Less than 30 mL/minute including patients on hemodialysis | 135 mcg once weekly |
| Less than 30 mL/minute including patients on hemodialysis* | 135 mcg once weekly |
| |
2.7 Preparation and Administration
Preparation and administration in adults: After proper training in subcutaneous injection, a patient may subcutaneously self-inject with PEGASYS if a healthcare provider determines that it is appropriate [see Instructions for Use]. Visually inspect PEGASYS for particulate matter and discoloration before administration (do not use if particulate matter is visible or product is discolored).
Table 6 displays the recommended volume of PEGASYS to be administered for the single-dose vial and prefilled syringe presentations for the different dosages recommendations (i.e., 180, 135, or 90 mcg once weekly). Discard the unused portion of PEGASYS in single-use vials or prefilled syringes in excess of the labeled volume.
| Recommended PEGASYS Dosage | PEGASYS Dosage Forms | |||
|---|---|---|---|---|
| 180 mcg/mL in a vial | 180 mcg/0.5 mL in a prefilled syringe* | |||
| 180 mcg | Use entire 1 mL | Use entire 0.5 mL | ||
| 135 mcg | Use 0.75 mL | Use 0.375 mL | ||
| 90 mcg | Use 0.5 mL | Use 0.25 mL | ||
| ||||
3. Dosage Forms and Strengths
4. Contraindications
PEGASYS is contraindicated in patients with:
- Known hypersensitivity reactions such as urticaria, angioedema, bronchoconstriction, anaphylaxis, or Stevens-Johnson syndrome to alpha interferons, including PEGASYS, or any of its components.
- Autoimmune hepatitis
- Hepatic decompensation (Child-Pugh score greater than 6 [class B and C]) in cirrhotic patients before treatment
- Hepatic decompensation with Child-Pugh score greater than or equal to 6 in cirrhotic CHC patients coinfected with HIV before treatment
PEGASYS is contraindicated in neonates and infants because it contains benzyl alcohol. Benzyl alcohol is associated with an increased incidence of neurologic and other complications which are sometimes fatal in neonates and infants.
When PEGASYS is used in combination with other HCV antiviral drugs, the contraindications applicable to those agents are applicable to combination therapies. PEGASYS combination treatment with ribavirin is contraindicated in women who are pregnant and men whose female partners are pregnant [See Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Refer to the prescribing information of the other HCV antiviral drugs, including ribavirin, for a list of their contraindications.
5. Warnings and Precautions
Refer to the prescribing information of the other HCV antiviral drugs, including ribavirin, for their Warnings and Precautions.
5.1 Pregnancy: Use with Ribavirin
Ribavirin may cause birth defects and/or death of the exposed fetus. Patients must avoid pregnancy (female patients or female partners of male patients) while taking PEGASYS and ribavirin combination therapy. Ribavirin therapy should not be started unless a confirmed negative pregnancy test has been obtained immediately prior to initiation of therapy. Women of childbearing potential and men must use two forms of effective contraception during treatment and for at least 6 months after treatment has concluded. Routine monthly pregnancy tests must be performed during this time [see Contraindications (4), Patient Counseling Information (17) and ribavirin labeling].
5.2 Neuropsychiatric Reactions
Life-threatening or fatal neuropsychiatric reactions may manifest in all patients receiving therapy with PEGASYS and include suicide, suicidal ideation, homicidal ideation, depression, relapse of drug addiction, and drug overdose. These reactions may occur in patients with and without previous psychiatric illness.
PEGASYS should be used with extreme caution in all patients who report a history of depression. Neuropsychiatric adverse events observed with alpha interferon treatment include aggressive behavior, psychoses, hallucinations, bipolar disorders, and mania. Physicians should monitor all patients for evidence of depression and other psychiatric symptoms. Patients should be advised to report any sign or symptom of depression or suicidal ideation to their prescribing physicians. In severe cases, therapy should be stopped immediately and psychiatric intervention instituted [see Boxed Warning, Adverse Reactions (6.1) and Dosage and Administration (2.6)].
5.3 Cardiovascular Disorders
Hypertension, supraventricular arrhythmias, chest pain, and myocardial infarction have been observed in patients treated with PEGASYS. PEGASYS should be administered with caution to patients with pre-existing cardiac disease. Because cardiac disease may be worsened by ribavirin-induced anemia, patients with a history of significant or unstable cardiac disease should not receive PEGASYS/ribavirin [see ribavirin prescribing information].
5.4 Bone Marrow Suppression
PEGASYS suppresses bone marrow function and may result in severe cytopenias. Ribavirin may potentiate the neutropenia and lymphopenia induced by alpha interferons including PEGASYS. Very rarely, alpha interferons may be associated with aplastic anemia. It is advised that complete blood counts (CBC) be obtained pre-treatment and monitored routinely during therapy [see ribavirin prescribing information].
PEGASYS/ribavirin should be used with caution in patients with baseline neutrophil counts less than 1,500 cells/mm3, with baseline platelet counts less than 90,000 cells/mm3 or baseline hemoglobin less than 10 g/dL. PEGASYS therapy should be discontinued, at least temporarily, in patients who develop severe decreases in neutrophil and/or platelet counts [see Dosage and Administration (2.6)].
Severe neutropenia and thrombocytopenia occur with a greater incidence in HIV coinfected patients than monoinfected patients and may result in serious infections or bleeding [see Adverse Reactions (6.1)].
Pancytopenia (marked decreases in RBCs, neutrophils and platelets) and bone marrow suppression have been reported in the literature to occur within 3 to 7 weeks after the concomitant administration of pegylated interferon/ribavirin and azathioprine. In this limited number of patients (n=8), myelotoxicity was reversible within 4 to 6 weeks upon withdrawal of both HCV antiviral therapy and concomitant azathioprine and did not recur upon reintroduction of either treatment alone. PEGASYS, ribavirin, and azathioprine should be discontinued for pancytopenia, and pegylated interferon/ribavirin should not be re-introduced with concomitant azathioprine.
5.5 Autoimmune Disorders
Development or exacerbation of autoimmune disorders including myositis, hepatitis, thrombotic thrombocytopenic purpura, idiopathic thrombocytopenic purpura, psoriasis, rheumatoid arthritis, interstitial nephritis, thyroiditis, and systemic lupus erythematosus have been reported in patients receiving alpha interferon. PEGASYS should be used with caution in patients with autoimmune disorders [see Boxed Warning].
5.6 Endocrine Disorders
PEGASYS causes or aggravates hypothyroidism and hyperthyroidism. Hyperglycemia, hypoglycemia, and diabetes mellitus have been observed to develop in patients treated with PEGASYS. Patients with these conditions at baseline who cannot be effectively treated by medication should not begin PEGASYS therapy. Patients who develop these conditions during treatment and cannot be controlled with medication may require discontinuation of PEGASYS therapy.
5.7 Ophthalmologic Disorders
Decrease or loss of vision, retinopathy including macular edema, retinal artery or vein thrombosis, retinal hemorrhages and cotton wool spots, optic neuritis, papilledema and serous retinal detachment are induced or aggravated by treatment with PEGASYS or other alpha interferons. All patients should receive an eye examination at baseline. Patients with pre-existing ophthalmologic disorders (e.g., diabetic or hypertensive retinopathy) should receive periodic ophthalmologic exams during interferon alpha treatment. Any patient who develops ocular symptoms should receive a prompt and complete eye examination. PEGASYS treatment should be discontinued in patients who develop new or worsening ophthalmologic disorders.
5.8 Cerebrovascular Disorders
Ischemic and hemorrhagic cerebrovascular events have been observed in patients treated with interferon alfa-based therapies, including PEGASYS. Events occurred in patients with few or no reported risk factors for stroke, including patients less than 45 years of age. Because these are spontaneous reports, estimates of frequency cannot be made and a causal relationship between interferon alfa-based therapies and these events is difficult to establish [see Boxed Warning].
5.9 Hepatic Failure and Hepatitis Exacerbations
Chronic hepatitis C (CHC) patients with cirrhosis may be at risk of hepatic decompensation and death when treated with alpha interferons, including PEGASYS. Cirrhotic CHC patients coinfected with HIV receiving highly active antiretroviral therapy (HAART) and interferon alfa-2a with or without ribavirin appear to be at increased risk for the development of hepatic decompensation compared to patients not receiving HAART. In Study 7 [see Clinical Studies (14.3)], among 129 CHC/HIV cirrhotic subjects receiving HAART, 14 (11%) of these subjects across all treatment arms developed hepatic decompensation resulting in 6 deaths. All 14 subjects were on NRTIs, including stavudine, didanosine, abacavir, zidovudine, and lamivudine. These small numbers of patients do not permit discrimination between specific NRTIs for the associated risk. During treatment, patients' clinical status and hepatic function should be closely monitored, and PEGASYS/ribavirin treatment should be immediately discontinued in patients with hepatic decompensation [see Contraindications (4)].
Exacerbations of hepatitis during hepatitis B therapy are not uncommon and are characterized by transient and potentially severe increases in serum ALT. Chronic hepatitis B subjects experienced transient acute exacerbations (flares) of hepatitis B (ALT elevation greater than 10-fold higher than the upper limit of normal) during PEGASYS treatment (12% and 18%) and post-treatment (7% and 12%) in HBeAg-negative and HBeAg-positive subjects, respectively. Marked transaminase flares while on PEGASYS therapy have been accompanied by other liver test abnormalities. Patients experiencing ALT flares should receive more frequent monitoring of liver function. PEGASYS dose reduction should be considered in patients experiencing transaminase flares. If ALT increases are progressive despite reduction of PEGASYS dose or are accompanied by increased bilirubin or evidence of hepatic decompensation, PEGASYS should be immediately discontinued [see Adverse Reactions (6.1) and Dosage and Administration (2.6)].
5.10 Pulmonary Disorders
Dyspnea, pulmonary infiltrates, pneumonia, bronchiolitis obliterans, interstitial pneumonitis, pulmonary hypertension and sarcoidosis, some resulting in respiratory failure and/or patient deaths, may be induced or aggravated by PEGASYS or alpha interferon therapy. Recurrence of respiratory failure has been observed with interferon rechallenge. PEGASYS combination treatment should be suspended in patients who develop pulmonary infiltrates or pulmonary function impairment. Patients who resume interferon treatment should be closely monitored.
5.11 Infections
While fever may be associated with the flu-like syndrome reported commonly during interferon therapy, other causes of high or persistent fever must be ruled out, particularly in patients with neutropenia. Serious and severe infections (bacterial, viral, or fungal), some fatal, have been reported during treatment with alpha interferons including PEGASYS. Appropriate anti-infective therapy should be started immediately and discontinuation of therapy should be considered [see Boxed Warning].
5.12 Colitis
Ulcerative and hemorrhagic/ischemic colitis, sometimes fatal, have been observed within 12 weeks of starting alpha interferon treatment. Abdominal pain, bloody diarrhea, and fever are the typical manifestations of colitis. PEGASYS should be discontinued immediately if these symptoms develop. The colitis usually resolves within 1 to 3 weeks of discontinuation of alpha interferon.
5.13 Pancreatitis
Pancreatitis, sometimes fatal, has occurred during alpha interferon and ribavirin treatment. PEGASYS/ribavirin should be suspended if symptoms or signs suggestive of pancreatitis are observed. PEGASYS/ribavirin should be discontinued in patients diagnosed with pancreatitis.
5.14 Hypersensitivity
Severe acute hypersensitivity reactions (e.g., urticaria, angioedema, bronchoconstriction, and anaphylaxis) have been observed during alpha interferon and ribavirin therapy. If such reaction occurs, therapy with PEGASYS/ribavirin should be discontinued and appropriate medical therapy immediately instituted. Serious skin reactions including vesiculobullous eruptions, reactions in the spectrum of Stevens-Johnson Syndrome (erythema multiforme major) with varying degrees of skin and mucosal involvement and exfoliative dermatitis (erythroderma) have been reported in patients receiving PEGASYS with and without ribavirin. Patients developing signs or symptoms of severe skin reactions must discontinue therapy [see Adverse Reactions (6.2)].
5.15 Impact on Growth in Pediatric Patients
Growth inhibition was observed in CHC pediatric subjects 5 to 17 years of age during combination therapy for up to 48 weeks with PEGASYS plus ribavirin. At the end of treatment, 43% of subjects were more than 15 percentiles below their baseline weight curve, and 25% of subjects were more than 15 percentiles below their baseline height curve. At the end of 2 years follow-up after treatment, most subjects had returned to baseline normative curve percentiles for weight and height; 16% of subjects were more than 15 percentiles below their baseline weight curve and 11% were more than 15 percentiles below their baseline height curve.
The available longer term data on subjects who were followed up to 6 years post-treatment are too limited to determine the risk of reduced adult height in some patients [see Clinical Trials Experience (6.1)].
Growth inhibition was also observed in CHB pediatric subjects 3 to 17 years of age during therapy with PEGASYS lasting up to 48 weeks. At Week 48 of treatment 11% of subjects were more than 15 percentiles below their baseline weight curve and 6% were more than 15 percentiles below their baseline height curve. At 24 weeks after the end of treatment, 12% of subjects were more than 15 percentiles below their baseline weight curve and 12% were more than 15 percentiles below their baseline height curve. No data are available on longer term follow-up post-treatment in these patients [see Clinical Trials Experience (6.1)].
5.16 Peripheral Neuropathy
Peripheral neuropathy has been reported when alpha interferons were given in combination with telbivudine. In one clinical trial, an increased risk and severity of peripheral neuropathy was observed with the combination use of telbivudine and PEGASYS as compared to telbivudine alone. The safety and efficacy of telbivudine in combination with interferons for the treatment of CHB have not been demonstrated.
5.17 Laboratory Tests
Before beginning PEGASYS or PEGASYS combination therapy, standard hematological and biochemical laboratory tests are recommended for all patients. Pregnancy screening for women of childbearing potential must be performed. Patients who have pre-existing cardiac abnormalities should have electrocardiograms administered before treatment with PEGASYS/ribavirin.
After initiation of therapy, hematological tests should be performed at 2 weeks and 4 weeks and biochemical tests should be performed at 4 weeks. Additional testing should be performed periodically during therapy. In adult clinical studies, the CBC (including hemoglobin level and white blood cell and platelet counts) and chemistries (including liver function tests and uric acid) were measured at 1, 2, 4, 6, and 8 weeks, and then every 4 to 6 weeks or more frequently if abnormalities were found. In a pediatric clinical trial, hematological and chemistry assessments were at 1, 3, 5, and 8 weeks, then every 4 weeks. Thyroid stimulating hormone (TSH) was measured every 12 weeks. Monthly pregnancy testing should be performed during combination therapy and for 6 months after discontinuing therapy.
The entrance criteria used for the clinical studies of PEGASYS may be considered as a guideline to acceptable baseline values for initiation of treatment:
- Platelet count greater than or equal to 90,000 cells/mm3 (as low as 75,000 cells/mm3 in HCV subjects with cirrhosis or 70,000 cells/mm3 in subjects with CHC and HIV)
- Absolute neutrophil count (ANC) greater than or equal to 1,500 cells/mm3
- Serum creatinine concentration less than 1.5 × upper limit of normal
- TSH and T4 within normal limits or adequately controlled thyroid function
- CD4+ cell count greater than or equal to 200 cells/mm3 or CD4+ cell count greater than or equal to 100 cells/mm3 but less than 200 cells/mm3 and HIV-1 RNA less than 5,000 copies/mL in subjects coinfected with HIV
- Hemoglobin greater than or equal to 12 g/dL for women and greater than or equal to 13 g/dL for men in CHC monoinfected subjects
- Hemoglobin greater than or equal to 11 g/dL for women and greater than or equal to 12 g/dL for men in subjects with CHC and HIV
6. Adverse Reactions
In clinical trials, a broad variety of serious adverse reactions were observed in 1,010 subjects who received PEGASYS at doses of 180 mcg for 48 weeks, alone or in combination with COPEGUS® [see Boxed Warning and Warnings and Precautions (5)]. The most common life-threatening or fatal events induced or aggravated by PEGASYS and COPEGUS include depression, suicide, relapse of drug abuse/overdose, and bacterial infections, each occurring at a frequency of less than 1%. Hepatic decompensation occurred in 2% (10/574) of CHC/HIV subjects [see Warnings and Precautions (5.9)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying and controlled conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates observed in clinical practice.
Chronic Hepatitis C
Adult Subjects
In all hepatitis C studies, one or more serious adverse reactions occurred in 10% of CHC monoinfected subjects and in 19% of CHC/HIV subjects receiving PEGASYS alone or in combination with COPEGUS. The most common serious adverse reactions (3% in CHC and 5% in CHC/HIV) was bacterial infection (e.g., sepsis, osteomyelitis, endocarditis, pyelonephritis, pneumonia). Other SAEs occurred at a frequency of less than 1% and included: suicide, suicidal ideation, aggression, anxiety, drug abuse and drug overdose, angina, hepatic dysfunction, fatty liver, cholangitis, arrhythmia, diabetes mellitus, autoimmune phenomena (e.g., hyperthyroidism, hypothyroidism, sarcoidosis, systemic lupus erythematosus, rheumatoid arthritis), peripheral neuropathy, aplastic anemia, peptic ulcer, gastrointestinal bleeding, pancreatitis, colitis, corneal ulcer, pulmonary embolism, coma, myositis, cerebral hemorrhage, thrombotic thrombocytopenic purpura, psychotic disorder, and hallucination.
In clinical trials, 98 to 99 percent of subjects experienced one or more adverse reactions. For hepatitis C subjects, the most commonly reported adverse reactions were psychiatric reactions, including depression, insomnia, irritability, anxiety, and flu-like symptoms such as fatigue, pyrexia, myalgia, headache, and rigors. Other common reactions were anorexia, nausea and vomiting, diarrhea, arthralgias, injection site reactions, alopecia, and pruritus. Table 7 displays pooled rates of adverse reactions occurring in greater than 5% of subjects in the PEGASYS monotherapy and PEGASYS/COPEGUS combination therapy clinical trials.
Overall 11% of CHC monoinfected subjects receiving 48 weeks of therapy with PEGASYS either alone or in combination with COPEGUS discontinued therapy; 16% of CHC/HIV coinfected subjects discontinued therapy. The most common reasons for discontinuation of therapy were psychiatric, flu-like syndrome (e.g., lethargy, fatigue, headache), dermatologic and gastrointestinal disorders, and laboratory abnormalities (thrombocytopenia, neutropenia, and anemia).
Overall 39% of subjects with CHC or CHC/HIV required modification of PEGASYS and/or COPEGUS therapy. The most common reasons for dose modification of PEGASYS in CHC and CHC/HIV subjects was for neutropenia (20% and 27%, respectively) and thrombocytopenia (4% and 6%, respectively). The most common reason for dose modification of COPEGUS in CHC and CHC/HIV subjects was anemia (22% and 16%, respectively). PEGASYS dose was reduced in 12% of subjects receiving 1000 mg to 1200 mg COPEGUS for 48 weeks and in 7% of subjects receiving 800 mg COPEGUS for 24 weeks. COPEGUS dose was reduced in 21% of subjects receiving 1000 mg to 1200 mg COPEGUS for 48 weeks and in 12% of subjects receiving 800 mg COPEGUS for 24 weeks.
Chronic hepatitis C monoinfected subjects treated for 24 weeks with PEGASYS and 800 mg COPEGUS were observed to have lower incidence of serious adverse reactions (3% vs. 10%), Hgb less than 10 g/dL (3% vs. 15%), dose modification of PEGASYS (30% vs. 36%) and COPEGUS (19% vs. 38%) and of withdrawal from treatment (5% vs. 15%) compared to subjects treated for 48 weeks with PEGASYS and 1000 mg or 1200 mg COPEGUS. The overall incidence of adverse reactions appeared to be similar in the two treatment groups.
| CHC Monotherapy (Pooled Studies 1-3) | CHC Combination Therapy (Study 4) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Body System | PEGASYS 180 mcg 48 week* | ROFERON-A Either 3 MIU† or 6/3 MIU† of ROFERON-A 48 week* | PEGASYS 180 mcg + 1000 mg or 1200 mg COPEGUS 48 week‡ | Intron® A + 1000 mg or 1200 mg Rebetol ®
48 week‡ | ||||||||||||
| N=559 | N=554 | N=451 | N=443 | |||||||||||||
| % | % | % | % | |||||||||||||
| Application Site Disorders | ||||||||||||||||
| Injection site reaction | 22 | 18 | 23 | 16 | ||||||||||||
| Endocrine Disorders | ||||||||||||||||
| Hypothyroidism | 3 | 2 | 4 | 5 | ||||||||||||
| Flu-like Symptoms and Signs | ||||||||||||||||
| Fatigue/Asthenia | 56 | 57 | 65 | 68 | ||||||||||||
| Pyrexia | 37 | 41 | 41 | 55 | ||||||||||||
| Rigors | 35 | 44 | 25 | 37 | ||||||||||||
| Pain | 11 | 12 | 10 | 9 | ||||||||||||
| Gastrointestinal | ||||||||||||||||
| Nausea/Vomiting | 24 | 33 | 25 | 29 | ||||||||||||
| Diarrhea | 16 | 16 | 11 | 10 | ||||||||||||
| Abdominal pain | 15 | 15 | 8 | 9 | ||||||||||||
| Dry mouth | 6 | 3 | 4 | 7 | ||||||||||||
| Dyspepsia | <1 | 1 | 6 | 5 | ||||||||||||
| Hematologic§ | ||||||||||||||||
| Lymphopenia | 3 | 5 | 14 | 12 | ||||||||||||
| Anemia | 2 | 1 | 11 | 11 | ||||||||||||
| Neutropenia | 21 | 8 | 27 | 8 | ||||||||||||
| Thrombocytopenia | 5 | 2 | 5 | <1 | ||||||||||||
| Metabolic and Nutritional | ||||||||||||||||
| Anorexia | 17 | 17 | 24 | 26 | ||||||||||||
| Weight decrease | 4 | 3 | 10 | 10 | ||||||||||||
| Musculoskeletal, Connective Tissue and Bone | ||||||||||||||||
| Myalgia | 37 | 38 | 40 | 49 | ||||||||||||
| Arthralgia | 28 | 29 | 22 | 23 | ||||||||||||
| Back pain | 9 | 10 | 5 | 5 | ||||||||||||
| Neurological | ||||||||||||||||
| Headache | 54 | 58 | 43 | 49 | ||||||||||||
| Dizziness (excluding vertigo) | 16 | 12 | 14 | 14 | ||||||||||||
| Memory impairment | 5 | 4 | 6 | 5 | ||||||||||||
Resistance Mechanism Disorders | ||||||||||||||||
| Overall | 10 | 6 | 12 | 10 | ||||||||||||
| Psychiatric | ||||||||||||||||
| Irritability/Anxiety/Nervousness | 19 | 22 | 33 | 38 | ||||||||||||
| Insomnia | 19 | 23 | 30 | 37 | ||||||||||||
| Depression | 18 | 19 | 20 | 28 | ||||||||||||
| Concentration impairment | 8 | 10 | 10 | 13 | ||||||||||||
| Mood alteration | 3 | 2 | 5 | 6 | ||||||||||||
| Respiratory, Thoracic and Mediastinal | ||||||||||||||||
| Dyspnea | 4 | 2 | 13 | 14 | ||||||||||||
| Cough | 4 | 3 | 10 | 7 | ||||||||||||
| Dyspnea exertional | <1 | <1 | 4 | 7 | ||||||||||||
| Skin and Subcutaneous Tissue | ||||||||||||||||
| Alopecia | 23 | 30 | 28 | 33 | ||||||||||||
| Pruritus | 12 | 8 | 19 | 18 | ||||||||||||
| Dermatitis | 8 | 3 | 16 | 13 | ||||||||||||
| Dry skin | 4 | 3 | 10 | 13 | ||||||||||||
| Rash | 5 | 4 | 8 | 5 | ||||||||||||
| Sweating increased | 6 | 7 | 6 | 5 | ||||||||||||
| Eczema | 1 | 1 | 5 | 4 | ||||||||||||
| Visual Disorders | ||||||||||||||||
| Vision blurred | 4 | 2 | 5 | 2 | ||||||||||||
| ||||||||||||||||
Pediatric Subjects
In a clinical trial with 114 pediatric subjects (5 to 17 years of age) treated with PEGASYS alone or in combination with COPEGUS, dose modifications were required in approximately one-third of subjects, most commonly for neutropenia and anemia. In general, the safety profile observed in pediatric subjects was similar to that seen in adults. In the pediatric study, the most prevalent adverse events in subjects treated with combination therapy for up to 48 weeks with PEGASYS and COPEGUS were influenza-like illness (91%), upper respiratory tract infection (60%), headache (64%), gastrointestinal disorder (56%), skin disorder (47%), and injection-site reaction (45%). Seven subjects receiving combination PEGASYS and COPEGUS treatment for 48 weeks discontinued therapy for safety reasons (depression, psychiatric evaluation abnormal, transient blindness, retinal exudates, hyperglycemia, type 1 diabetes mellitus, and anemia). Most of the adverse events reported in the study were mild or moderate in severity. Severe adverse events were reported in 2 subjects in the PEGASYS plus COPEGUS combination therapy group (hyperglycemia and cholecystectomy).
| Study NV17424 | |||||||
|---|---|---|---|---|---|---|---|
| System Organ Class | PEGASYS 180 mcg/1.73 m2 × BSA + COPEGUS 15 mg/kg (N=55) | PEGASYS 180 mcg/1.73 m2 × BSA + Placebo†
(N=59) | |||||
| % | % | ||||||
| General disorders and administration site conditions | |||||||
| Influenza like illness | 91 | 81 | |||||
| Injection site reaction | 44 | 42 | |||||
| Fatigue | 25 | 20 | |||||
| Irritability | 24 | 14 | |||||
| Gastrointestinal disorders | |||||||
| Gastrointestinal disorder | 49 | 44 | |||||
| Nervous system disorders | |||||||
| Headache | 51 | 39 | |||||
| Skin and subcutaneous tissue disorders | |||||||
| Rash | 15 | 10 | |||||
| Pruritus | 11 | 12 | |||||
| Musculoskeletal, connective tissue and bone disorders | |||||||
| Musculoskeletal pain | 35 | 29 | |||||
| Psychiatric disorders | |||||||
| Insomnia | 9 | 12 | |||||
| Metabolism and nutrition disorders | |||||||
| Decreased appetite | 11 | 14 | |||||
| |||||||
In pediatric subjects randomized to combination therapy, the incidence of most adverse reactions were similar for the entire treatment period (up to 48 weeks plus 24 weeks follow-up) in comparison to the first 24 weeks, and increased only slightly for headache, gastrointestinal disorder, irritability and rash. The majority of adverse reactions occurred in the first 24 weeks of treatment.
Growth inhibition in CHC pediatric subjects [see Warnings and Precautions (5.15)].
Pediatric subjects treated with PEGASYS plus ribavirin combination therapy showed a delay in weight and height increases up to 48 weeks of therapy compared with baseline. Both weight and height for age z-scores as well as the percentiles of the normative population for subject weight and height decreased during treatment. At the end of 2 years follow-up after treatment, most subjects had returned to baseline normative curve percentiles for weight (64th mean percentile at baseline, 60th mean percentile at 2 years post-treatment) and height (54th mean percentile at baseline, 56th mean percentile at 2 years post-treatment). At the end of treatment, 43% (23 of 53) of subjects experienced a weight percentile decrease of more than 15 percentiles, and 25% (13 of 53) experienced a height percentile decrease of more than 15 percentiles on the normative growth curves. At 2 years post-treatment, 16% (6 of 38) of subjects were more than 15 percentiles below their baseline weight curve and 11% (4 of 38) were more than 15 percentiles below their baseline height curve.
Thirty-eight of the 114 subjects enrolled in the long-term follow-up study extending up to 6 years post-treatment. For most subjects, post-treatment recovery in growth at 2 years post-treatment was maintained to 6 years post-treatment.
CHC with HIV Coinfection (Adults)
The adverse reaction profile of coinfected subjects treated with PEGASYS/COPEGUS in Study 7 was generally similar to that shown for monoinfected subjects in Study 4 (Table 7). Events occurring more frequently in coinfected subjects were neutropenia (40%), anemia (14%), thrombocytopenia (8%), weight decrease (16%), and mood alteration (9%).
Chronic Hepatitis B
Adult Subjects
In clinical trials of 48 week treatment duration, the adverse reaction profile of PEGASYS in CHB was similar to that seen in CHC PEGASYS monotherapy use, except for exacerbations of hepatitis [see Warnings and Precautions (5.9)]. Six percent of PEGASYS-treated subjects in the hepatitis B studies experienced one or more serious adverse reactions.
The most common or important serious adverse reactions, all of which occurred at a frequency of less than or equal to 1%, in the hepatitis B studies were infections (sepsis, appendicitis, tuberculosis, influenza), hepatitis B flares, and thrombotic thrombocytopenic purpura.
One serious adverse reaction of anaphylactic shock occurred in a dose ranging study of 191 subjects in a subject taking a higher than the approved dose of PEGASYS.
The most commonly observed adverse reactions in the PEGASYS and lamivudine groups, respectively, were pyrexia (54% vs. 4%), headache (27% vs. 9%), fatigue (24% vs. 10%), myalgia (26% vs. 4%), alopecia (18% vs. 2%), and anorexia (16% vs. 3%).
Overall 5% of hepatitis B subjects discontinued PEGASYS therapy and 40% of subjects required modification of PEGASYS dose. The most common reason for dose modification in subjects receiving PEGASYS therapy was for laboratory abnormalities including neutropenia (20%), thrombocytopenia (13%), and ALT elevation (11%).
Pediatric Subjects
In a clinical trial with 111 subjects 3 to 17 years of age treated with PEGASYS for 48 weeks, the safety profile was consistent with that seen in adults with CHB and in pediatric subjects with CHC. The most commonly observed adverse reactions in PEGASYS-treated patients were pyrexia (51%), headache (21%), abdominal pain (17%), cough (15%), vomiting (15%), influenza-like illness (14%), alanine aminotransferase increased (10%), aspartate aminotransferase increased (10%), rash (10%), asthenia (9.0%), epistaxis (9.0%), nausea (9.0%), fatigue (8%), upper respiratory tract infection (8%), alopecia (6%), decreased appetite (6%), dizziness (6%), and nasopharyngitis (6%).
Growth inhibition in CHB pediatric subjects [see Warnings and Precautions (5.15)].
The mean changes from baseline in z-scores for height and weight for age were -0.07 and -0.21 for PEGASYS-treated subjects at Week 48. Comparable findings were observed in untreated patients at Week 48 (changes in z-scores for height and weight for age were -0.01 and -0.08, respectively). At Week 48 of PEGASYS treatment, a height or weight decrease of more than 15 percentiles on the normative growth curves was observed in 6% of subjects for height and 11% of subjects for weight. At 24 weeks after the end of PEGASYS treatment the percentage of subjects with decrease of more than 15 percentiles from baseline were 12% for height and 12% for weight. No data are available on longer term follow-up post-treatment in these subjects.
Laboratory Values
Adult Subjects
The laboratory test values observed in the hepatitis B trials (except where noted below) were similar to those seen in the PEGASYS monotherapy CHC trials.
Neutrophils
In the hepatitis C studies, decreases in neutrophil count below normal were observed in 95% of all subjects treated with PEGASYS either alone or in combination with COPEGUS. Severe potentially life-threatening neutropenia (ANC less than 500 cells/mm3) occurred in 5% of CHC subjects and 12% of CHC/HIV subjects receiving PEGASYS either alone or in combination with COPEGUS. Modification of PEGASYS dose for neutropenia occurred in 17% of subjects receiving PEGASYS monotherapy and 22% of subjects receiving PEGASYS/COPEGUS combination therapy. In the CHC/HIV subjects 27% required modification of interferon dosage for neutropenia. Two percent of subjects with CHC and 10% of subjects with CHC/HIV required permanent reductions of PEGASYS dosage and less than 1% required permanent discontinuation. Median neutrophil counts return to pre-treatment levels 4 weeks after cessation of therapy [see Dosage and Administration (2.6)].
Lymphocytes
Decreases in lymphocyte count are induced by interferon alpha therapy. PEGASYS plus COPEGUS combination therapy induced decreases in median total lymphocyte counts (56% in CHC and 40% in CHC/HIV, with median decrease of 1170 cells/mm3 in CHC and 800 cells/mm3 in CHC/HIV). In the hepatitis C studies, lymphopenia was observed during both monotherapy (81%) and combination therapy with PEGASYS and COPEGUS (91%). Severe lymphopenia (less than 500 cells/mm3) occurred in approximately 5% of all monotherapy subjects and 14% of all combination PEGASYS and COPEGUS therapy recipients. Dose adjustments were not required by protocol. The clinical significance of the lymphopenia is not known.
In CHC with HIV coinfection, CD4 counts decreased by 29% from baseline (median decrease of 137 cells/mm3) and CD8 counts decreased by 44% from baseline (median decrease of 389 cells/mm3) in the PEGASYS plus COPEGUS combination therapy arm. Median lymphocyte CD4 and CD8 counts return to pre-treatment levels after 4 to 12 weeks of the cessation of therapy. CD4% did not decrease during treatment.
Platelets
In the hepatitis C studies, platelet counts decreased in 52% of CHC subjects and 51% of CHC/HIV subjects treated with PEGASYS alone (respectively median decrease of 41% and 35% from baseline), and in 33% of CHC subjects and 47% of CHC/HIV subjects receiving combination therapy with COPEGUS (median decrease of 30% from baseline). Moderate to severe thrombocytopenia (less than 50,000 cells/mm3) was observed in 4% of CHC and 8% of CHC/HIV subjects. Median platelet counts return to pre-treatment levels 4 weeks after the cessation of therapy.
Hemoglobin
In the hepatitis C studies, the hemoglobin concentration decreased below 12 g/dL in 17% (median Hgb reduction of 2.2 g/dL) of monotherapy and 52% (median Hgb reduction of 3.7 g/dL) of combination therapy subjects. Severe anemia (Hgb less than 10 g/dL) was encountered in 13% of all subjects receiving combination therapy and in 2% of CHC subjects and 8% of CHC/HIV subjects receiving PEGASYS monotherapy. Dose modification for anemia in COPEGUS recipients treated for 48 weeks occurred in 22% of CHC subjects and 16% of CHC/HIV subjects [see Dosage and Administration (2.6)].
Triglycerides
Triglyceride levels are elevated in subjects receiving alfa interferon therapy and were elevated in the majority of subjects participating in clinical studies receiving either PEGASYS alone or in combination with COPEGUS. Random levels greater than or equal to 400 mg/dL were observed in about 20% of CHC subjects. Severe elevations of triglycerides (greater than 1000 mg/dL) occurred in 2% of CHC monoinfected subjects.
In HCV/HIV coinfected subjects, fasting levels greater than or equal to 400 mg/dL were observed in up to 36% of subjects receiving either PEGASYS alone or in combination with COPEGUS. Severe elevations of triglycerides (greater than 1000 mg/dL) occurred in 7% of coinfected subjects.
ALT Elevations
Chronic Hepatitis C
One percent of subjects in the hepatitis C trials experienced marked elevations (5- to 10-fold above the upper limit of normal) in ALT levels during treatment and follow-up. These transaminase elevations were on occasion associated with hyperbilirubinemia and were managed by dose reduction or discontinuation of study treatment. Liver function test abnormalities were generally transient. One case was attributed to autoimmune hepatitis, which persisted beyond study medication discontinuation [see Dosage and Administration (2.6)].
Chronic Hepatitis B
Transient ALT elevations are common during hepatitis B therapy with PEGASYS. Twenty-five percent and 27% of subjects experienced elevations of 5 to 10 × ULN and 12% and 18% had elevations of greater than 10 × ULN during treatment of HBeAg negative and HBeAg positive disease, respectively. Flares have been accompanied by elevations of total bilirubin and alkaline phosphatase and less commonly with prolongation of PT and reduced albumin levels. Eleven percent of subjects had dose modifications due to ALT flares and less than 1% of subjects were withdrawn from treatment [see Warnings and Precautions (5.9) and Dosage and Administration (2.6)].
ALT flares of 5 to 10 × ULN occurred in 13% and 16% of subjects, while ALT flares of greater than 10 × ULN occurred in 7% and 12% of subjects in HBeAg-negative and HBeAg-positive disease, respectively, after discontinuation of PEGASYS therapy.
Thyroid Function
PEGASYS alone or in combination with COPEGUS was associated with the development of abnormalities in thyroid laboratory values, some with associated clinical manifestations. In the hepatitis C studies, hypothyroidism or hyperthyroidism requiring treatment, dose modification or discontinuation occurred in 4% and 1% of PEGASYS treated subjects and 4% and 2% of PEGASYS and COPEGUS treated subjects, respectively. Approximately half of the subjects, who developed thyroid abnormalities during PEGASYS treatment, still had abnormalities during the follow-up period [see Warnings and Precautions (5.6)].
Pediatric Subjects
Decreases in hemoglobin, neutrophils and platelets may require dose reduction or permanent discontinuation from treatment in pediatric subjects [see Dosage and Administration (2.3, 2.5, 2.6)]. Most laboratory abnormalities noted during the CHC clinical trial (Table 9) returned to baseline levels shortly after completion of treatment.
| Laboratory Parameter | PEGASYS 180 mcg/1.73 m2 × BSA + COPEGUS 15 mg/kg (N=55) | PEGASYS 180 mcg/1.73 m2 × BSA + Placebo*
(N=59) |
|---|---|---|
| Neutrophils (cells/mm3) | ||
| 1,000 - <1,500 | 31% | 39% |
| 750 - <1,000 | 27% | 17% |
| 500 - <750 | 25% | 15% |
| <500 | 7% | 5% |
| Platelets (cells/mm3) | ||
| 75,000 - <100,000 | 4% | 2% |
| 50,000 - <75,000 | 0% | 2% |
| < 50,000 | 0% | 0% |
| Hemoglobin (g/dL) | ||
| 8.5-<10 | 7% | 3% |
| <8.5 | 0% | 0% |
| ||
In patients randomized to combination therapy, the incidence of abnormalities during the entire treatment phase (up to 48 weeks plus 24 weeks follow-up) in comparison to the first 24 weeks increased slightly for neutrophils between 500 and 1,000 cells/mm3 and hemoglobin values between 8.5 and 10 g/dL. The majority of hematologic abnormalities occurred in the first 24 weeks of treatment.
The hematologic laboratory abnormalities observed in the CHB pediatric trial were similar to those observed in the CHC pediatric trial.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to peginterferon alfa-2a in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Chronic Hepatitis C
Nine percent (71/834) of subjects treated with PEGASYS with or without COPEGUS developed binding antibodies to interferon alfa-2a, as assessed by an ELISA assay. Three percent of subjects (25/835) receiving PEGASYS with or without COPEGUS, developed low-titer neutralizing antibodies (using an assay with a sensitivity of 100 INU/mL).
Chronic Hepatitis B
Twenty-nine percent (42/143) of hepatitis B subjects treated with PEGASYS for 24 weeks developed binding antibodies to interferon alfa-2a, as assessed by an ELISA assay. Thirteen percent of subjects (19/143) receiving PEGASYS developed low-titer neutralizing antibodies (using an assay with a sensitivity of 100 INU/mL).
The clinical and pathological significance of the appearance of serum neutralizing antibodies is unknown. No apparent correlation of antibody development to clinical response or adverse events was observed. The percentage of subjects whose test results were considered positive for antibodies is highly dependent on the sensitivity and specificity of the assays.
6.3 Postmarketing Experience
The following adverse reactions have been identified and reported during post-approval use of PEGASYS therapy. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: pure red cell aplasia
Ear and labyrinth disorders: hearing impairment, hearing loss
Gastrointestinal disorders: tongue pigmentation
Immune system disorders: liver graft rejection and renal graft rejection [see Use in Specific Populations (8.8)]
Infections and infestations: limb abscess
Metabolism and nutrition disorders: dehydration
Skin and subcutaneous tissue disorders: serious skin reactions
Neurological: seizures
7. Drug Interactions
7.1 Drugs Metabolized by Cytochrome P450
There was no effect on the pharmacokinetics of representative drugs metabolized by CYP 2C9, CYP 2C19, CYP 2D6 or CYP 3A4.
Treatment with PEGASYS once weekly for 4 weeks in healthy subjects was associated with an inhibition of P450 1A2 and a 25% increase in theophylline AUC.
7.2 Theophylline
Treatment with PEGASYS once weekly for 4 weeks in healthy subjects was associated with an inhibition of P450 1A2 and a 25% increase in theophylline AUC. Theophylline serum levels should be monitored and appropriate dose adjustments considered for patients given both theophylline and PEGASYS.
7.3 Methadone
In a PK study of HCV subjects concomitantly receiving methadone, treatment with PEGASYS once weekly for 4 weeks was associated with methadone levels that were 10% to 15% higher than at baseline. The clinical significance of this finding is unknown; however, patients should be monitored for the signs and symptoms of methadone toxicity.
The pharmacokinetics of concomitant administration of methadone and PEGASYS were evaluated in 24 PEGASYS naïve CHC subjects (15 male, 9 female) who received 180 mcg PEGASYS subcutaneously weekly. All subjects were on stable methadone maintenance therapy (median dose 95 mg, range 30 mg to 150 mg) prior to receiving PEGASYS. Mean methadone PK parameters were 10% to 15% higher after 4 weeks of PEGASYS treatment as compared to baseline. Methadone did not significantly alter the PK of PEGASYS as compared to a PK study of 6 chronic hepatitis C subjects not receiving methadone.
7.4 Nucleoside Analogues
NRTIs
In Study 7 among the CHC/HIV coinfected cirrhotic subjects receiving NRTIs cases of hepatic decompensation (some fatal) were observed [see Warnings and Precautions (5.9)].
Patients receiving PEGASYS/ribavirin in combination with other HCV antiviral drugs and NRTIs should be closely monitored for treatment associated toxicities. Physicians should refer to prescribing information for other HCV antiviral drugs and the respective NRTIs for guidance regarding toxicity management. In addition, dose reduction or discontinuation of PEGASYS, ribavirin or both, should also be considered if worsening toxicities are observed [see Warnings and Precautions (5.3, 5.9) and Dosage and Administration (2.6)].
Zidovudine
In Study 7, subjects who were administered zidovudine in combination with PEGASYS/COPEGUS developed severe neutropenia (ANC less than 500 cells/mm3) and severe anemia (hemoglobin less than 8 g/dL) more frequently than similar subjects not receiving zidovudine (neutropenia 15% vs. 9%) (anemia 5% vs. 1%). Discontinuation of zidovudine should be considered as medically appropriate. Dose reduction or discontinuation of PEGASYS, ribavirin or both should also be considered if worsening clinical toxicities are observed, including hepatic decompensation (e.g., Child-Pugh greater than 6).
Refer to the prescribing information for specific HCV antiviral drugs used in combination with PEGASYS for information on drug interaction potential.
8. Use in Specific Populations
8.1 Pregnancy
Pregnancy Exposure Registry – Use with Ribavirin
A Ribavirin Pregnancy Registry has been established to monitor maternal and fetal outcomes of pregnancies of female patients and female partners of male patients exposed to ribavirin during pregnancy or who become pregnant within 6 months following cessation of treatment with ribavirin. Healthcare providers and patients are encouraged to report such cases by calling 1-800-593-2214.
Risk Summary
There are no adequate and well-controlled studies of PEGASYS in pregnant women to inform a drug-associated risk. Based on animal reproduction studies, PEGASYS can cause fetal harm and should be assumed to have abortifacient potential. Non-pegylated interferon alfa-2a treatment caused abortion when given to pregnant rhesus monkeys (see Data). The background risk of major birth defects and miscarriage in the indicated population is 3% and 4-22%, respectively. In the U.S. general population, the estimated background risk for major birth defects and miscarriage in the clinically recognized pregnancies is 2-4% and 15-20%, respectively.
PEGASYS combination treatment with ribavirin is contraindicated in women who are pregnant and in the male partners of women who are pregnant [see Contraindications (4), Warnings and Precautions (5.1), and ribavirin labeling]. Significant teratogenic and/or embryocidal effects have been demonstrated in all animal species exposed to ribavirin [see ribavirin labeling].
Data
Animal Data – Groups of 8 or 9 pregnant rhesus monkeys were given non-pegylated interferon alfa-2a by daily intramuscular injection over days 22 to 70 of gestation at doses of 1, 5 and 25 million IU/day. Two, 3 and 6 monkeys aborted in the low, mid and high dose groups compared with 1 in the control group. Maternal toxicity, characterized by transient body weight loss, was seen at all dose levels. There were too few remaining pregnancies to assess teratogenic potential but no developmental abnormalities were observed in surviving fetuses.
8.2 Lactation
There is no information regarding the presence of peginterferon alfa-2a in human milk, the effects on the breastfed infant, or the effects on milk production. Because of the potential for adverse reactions from the drugs in nursing infants, a decision must be made whether to discontinue nursing or discontinue PEGASYS. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for PEGASYS and any potential adverse effects on the breastfed child from PEGASYS or from the underlying maternal condition.
The Centers for Disease Control and Prevention recommends that HIV-infected mothers not breastfeed their infants to avoid potential transmission of HIV; therefore, CHC- and CHB-infected mothers coinfected with HIV should not breastfeed their infants.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Females of reproductive potential must undergo pregnancy testing before initiation of treatment with PEGASYS or with PEGASYS in combination with ribavirin or with other HCV drugs [see Warnings and Precautions (5.17)].
Females of reproductive potential receiving PEGASYS in combination with ribavirin must have a routine pregnancy test performed monthly during treatment and for at least 6 months following treatment. Female partners of male patients receiving PEGASYS in combination with ribavirin must have a routine pregnancy test performed monthly during treatment and for at least 6 months posttherapy [see Warnings and Precautions (5.17), ribavirin prescribing information].
Contraception
Females
Because of the abortifacient potential of PEGASYS, females of reproductive potential should be advised to use effective contraception during therapy [see Warnings and Precautions (5.17)]. However, when receiving PEGASYS in combination with ribavirin, women of reproductive potential and their partners must use effective contraception during treatment and for at least 6 months after the last dose [see ribavirin prescribing information].
Infertility
Females
Based on its mechanism of action and studies in female monkeys, PEGASYS can cause disruption of the menstrual cycle [see Nonclinical Toxicology (13.1)]. No female fertility study has been performed.
8.4 Pediatric Use
PEGASYS is indicated for the treatment of CHC in pediatric patients 5 to 17 years of age and for the treatment of CHB in pediatric patients 3 to 17 years of age [see Indications and Usage (1.1), (1.2), Dosage and Administration (2.3), (2.5), Clinical Studies (14.1), (14.4)].
The use of PEGASYS for the treatment of pediatric patients 5 to 17 years of age with CHC is based on one clinical trial in 114 previously untreated CHC subjects 5 to 17 years of age with compensated liver disease and detectable HCV RNA [see Clinical Trials Experience (6.1), Clinical Studies (14.1)]. The safety and efficacy of PEGASYS in pediatric patients with CHC below the age of 5 years have not been established.
The use of PEGASYS for the treatment of pediatric patients 3 to 17 years of age with CHB is based on one clinical trial in 161 previously untreated CHB subjects 3 to 17 years of age of whom 111 were assigned to treatment with PEGASYS [see Clinical Trials Experience (6.1), Clinical Studies (14.4)]. PEGASYS has not been studied in pediatric CHB patients with liver cirrhosis and the safety and efficacy of PEGASYS in pediatric patients with CHB below the age of 3 years have not been established.
PEGASYS contains benzyl alcohol. In neonates and infants, benzyl alcohol has been reported to be associated with an increased incidence of neurological and other complications which are sometimes fatal in neonates and infants [see Contraindications (4)].
8.5 Geriatric Use
Younger patients have higher virologic response rates than older patients. Clinical studies of PEGASYS alone or in combination with COPEGUS did not include sufficient numbers of subjects aged 65 or over to determine whether they respond differently from younger subjects. Adverse reactions related to alpha interferons, such as CNS, cardiac, and systemic (e.g., flu-like) effects may be more severe in the elderly and caution should be exercised in the use of PEGASYS in this population. PEGASYS is excreted by the kidney, and the risk of toxic reactions to this therapy may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function. PEGASYS should be used with caution in patients with creatinine clearance less than or equal to 50 mL/min. The dose of PEGASYS should be reduced for patients with creatinine clearance less than 30 mL/min [see Dosage and Administration (2.6) and Use in Specific Populations (8.7)].
8.6 Hepatic Impairment
CHC patients with cirrhosis may be at risk of hepatic decompensation and death when treated with alpha interferons, including PEGASYS. During treatment, patients' clinical status and hepatic function should be closely monitored, and PEGASYS treatment should be immediately discontinued if decompensation (Child-Pugh score greater than or equal to 6) is observed [see Contraindications (4)]. Chronic hepatitis B subjects experienced transient acute exacerbations (flares) of hepatitis B (ALT elevation greater than 10-fold higher than the upper limit of normal) during PEGASYS treatment (12% and 18%) and post-treatment (7% and 12%) in HBeAg-negative and HBeAg-positive subjects, respectively.
8.7 Renal Impairment
Renal function should be evaluated in all patients prior to initiation of PEGASYS by estimating the patient's creatinine clearance.
A clinical trial evaluated treatment with PEGASYS and COPEGUS in 50 CHC subjects with moderate (creatinine clearance 30 – 50 mL/min) or severe (creatinine clearance less than 30 mL/min) renal impairment or end stage renal disease (ESRD) requiring chronic hemodialysis (HD). In 18 subjects with ESRD receiving chronic HD, PEGASYS was administered at a dose of 135 mcg once weekly. Dose reductions and temporary interruptions of PEGASYS (due to PEGASYS-related adverse reactions, mainly anemia) were observed in up to 22% ESRD/HD subjects during treatment; and 17% of these subjects discontinued PEGASYS due to PEGASYS-related adverse reactions. Only one-third of ESRD/HD subjects received PEGASYS for 48 weeks. Subjects with severe (n=14) or moderate (n=17) renal impairment received PEGASYS 180 mcg once weekly. PEGASYS discontinuation rates were 36% and 0% in subjects with severe and moderate renal impairment, respectively, compared to 0% discontinuation rate in subjects with normal renal function.
Based on the pharmacokinetic and safety results from this trial, patients with creatinine clearance less than 30 mL/min should receive a reduced dose of PEGASYS. In addition, patients with any degree of renal impairment should be carefully monitored for laboratory abnormalities (especially decreased hemoglobin) and adverse reactions, and should undergo careful monitoring of creatinine clearance. Patients with clinically significant laboratory abnormalities or adverse reactions which are persistently severe or worsening should have therapy withdrawn [see Dosage and Administration (2.6), Clinical Pharmacology (12.3)]. Refer to the prescribing information for specific HCV antiviral drugs used in combination with PEGASYS for information on use in patients with renal impairment.
10. Overdosage
There is limited experience with overdosage. The maximum dose received by any patient was 7 times the intended dose of PEGASYS (180 mcg/day for 7 days). There were no serious reactions attributed to overdosages. Weekly doses of up to 630 mcg have been administered to patients with cancer. Dose-limiting toxicities were fatigue, elevated liver enzymes, neutropenia, and thrombocytopenia. There is no specific antidote for PEGASYS. Hemodialysis and peritoneal dialysis are not effective.
11. Description
Peginterferon alfa-2a, is a covalent conjugate of recombinant alfa-2a interferon (approximate molecular weight [MW] 20,000 daltons) with a single branched bis-monomethoxy polyethylene glycol (PEG) chain (approximate MW 40,000 daltons). The PEG moiety is linked at a single site to the interferon alfa moiety via a stable amide bond to lysine. Peginterferon alfa-2a has an approximate molecular weight of 60,000 daltons. Interferon alfa-2a is produced using recombinant DNA technology in which a cloned human leukocyte interferon gene is inserted into and expressed in Escherichia coli.
PEGASYS is a sterile, preservative-free, colorless to slightly yellowish solution available as an injection and is administered subcutaneously.
Each vial of 180 mcg/mL peginterferon alfa-2a (expressed as the amount of interferon alfa-2a) also contains acetic acid (0.05 mg), benzyl alcohol (10 mg), polysorbate 80 (0.05 mg), sodium acetate trihydrate (2.62 mg), and sodium chloride (8 mg) at pH 6 ± 0.5.
Each prefilled syringe of 180 mcg/0.5 mL peginterferon alfa-2a (expressed as the amount of interferon alfa-2a) also contains acetic acid (0.0231 mg), benzyl alcohol (5 mg), polysorbate 80 (0.025 mg), sodium acetate trihydrate (1.3085 mg), and sodium chloride (4 mg) at pH 6 ± 0.5.
12. Clinical Pharmacology
12.1 Mechanism of Action
Pegylated recombinant human interferon alfa-2a is an inducer of the innate antiviral immune response [see Microbiology (12.4)].
12.2 Pharmacodynamics
PEGASYS stimulates the production of effector proteins such as serum neopterin and 2', 5'-oligoadenylate synthetase.
12.3 Pharmacokinetics
Maximal serum concentrations (Cmax) and AUC increased in a nonlinear dose related manner following administration of 90 to 270 mcg of PEGASYS. Maximal serum concentrations (Cmax) occur between 72 to 96 hours post-dose.
Week 48 mean trough concentrations (16 ng/mL; range 4 to 28) at 168 hours post-dose are approximately 2-fold higher than week 1 mean trough concentrations (9 ng/mL; range 0 to 15). Steady-state serum levels are reached within 5 to 8 weeks of once weekly dosing. The peak to trough ratio at week 48 is approximately 2. The mean systemic clearance in healthy subjects given PEGASYS was 94 mL/h, which is approximately 100-fold lower than that for interferon alfa-2a (ROFERON®-A). The mean terminal half-life after subcutaneous dosing in subjects with chronic hepatitis C was 160 hours (range 84 to 353 hours) compared to 5 hours (range 3.7 to 8.5 hours) for ROFERON-A.
Specific Populations
Gender and Age
PEGASYS administration yielded similar pharmacokinetics in male and female healthy subjects. The AUC was increased from 1295 to 1663 ng∙h/mL in subjects older than 62 years taking 180 mcg PEGASYS, but peak concentrations were similar (9 vs. 10 ng/mL) in those older and younger than 62 years.
Pediatric Patients
In a population pharmacokinetics study, 14 children 2 to 8 years of age with CHC received PEGASYS based on their body surface area (BSA of the child × 180 mcg/1.73 m2). The clearance of PEGASYS in children was nearly 4-fold lower compared to the clearance reported in adults.
Steady-state trough levels in children with the BSA-adjusted dosing were similar to trough levels observed in adults with 180 mcg fixed dosing. Time to reach the steady state in children is approximately 12 weeks, whereas in adults, steady state is reached within 5 to 8 weeks. In these children receiving the BSA adjusted dose, the mean exposure (AUC) during the dosing interval is predicted to be 25% to 70% higher than that observed in adults receiving 180 mcg fixed dosing.
Based on the population pharmacokinetic model including data from 30 pediatric CHB patients who received the BSA based dosing regimen [see Dosage and Administration (2.5)], AUC values in pediatric CHB patients were comparable with that observed in adults receiving 180 mcg dosing.
Renal Impairment
A clinical trial evaluated 50 CHC subjects with either moderate (creatinine clearance 30 to 50 mL/min) or severe (creatinine clearance less than 30 mL/min) renal impairment or end stage renal disease (ESRD) requiring chronic hemodialysis (HD). Subjects with moderate renal impairment receiving PEGASYS 180 mcg once weekly dose exhibited similar peginterferon alfa-2a plasma exposures compared to subjects with normal renal function (creatinine clearance greater than 80 mL/min) receiving the standard dose of PEGASYS. No PEGASYS dose adjustment is required for patients with mild or moderate renal impairment [see Dosage and Administration (2.6) and Use in Specific Populations (8.7)].
For subjects with severe renal impairment, peginterferon alfa-2a apparent clearance was 43% lower as compared to subjects with normal renal function. A reduced dose of 135 mcg once weekly PEGASYS is recommended in patients with severe renal impairment. This dose may result in 30% higher peginterferon alfa-2a exposure compared to that of the recommended dose for patients with normal renal function. Signs and symptoms of interferon toxicity should be closely monitored in patients with severe renal impairment and the dose reduced to 90 mcg once weekly as appropriate [see Dosage and Administration (2.6) and Use in Specific Populations (8.7)].
In 18 subjects with ESRD receiving chronic HD, PEGASYS was administered at a dose of 135 mcg once weekly. The apparent clearance of peginterferon alfa-2a was similar between subjects with ESRD and subjects with normal renal function. Despite a lower exposure to peginterferon alfa-2a with the 135 mcg dose, subjects with ESRD had a high rate of adverse events and discontinuations of PEGASYS in the trial. Therefore, a dose of 135 mcg once weekly should be used for patients with ESRD on HD. However, the potential for reduced efficacy and increased interferon toxicity in patients with ESRD receiving chronic HD should be closely monitored. The dose may be reduced to 90 mcg once weekly as appropriate [see Dosage and Administration (2.6) and Use in Specific Populations (8.7)].
12.4 Microbiology
Mechanism of Action
The biological activity of PEGASYS is derived from its recombinant human interferon α-2a moiety. Peginterferon α-2a binds to the human type 1 interferon receptor leading to receptor dimerization. Receptor dimerization activates multiple intracellular signal transduction pathways initially mediated by the JAK/STAT pathway. Given the diversity of cell types that respond to interferon α-2a, and the multiplicity of potential intracellular responses to interferon receptor activation, peginterferon α-2a is expected to have pleiotropic biological effects in the body.
Activity against HCV
Antiviral Activity in Cell Culture
In the stable HCV cell culture model system (HCV replicon), peginterferon α-2a inhibited HCV RNA replication, with an EC50 value of 0.1-3 ng/mL. The combination of peginterferon α-2a and ribavirin was more effective at inhibiting HCV RNA replication than either agent alone.
Activity against HBV
Antiviral Activity in Cell Culture and Animal Model
The antiviral activity of interferon α-2a (standard and pegylated) has been demonstrated using an HBV cell culture system and a mouse model. In human hepatoma cells (HepG2 and Huh7) transiently transfected with HBV, interferon α-2a (parent compound of PEGASYS) at a 1,000 IU/mL dose was active against all 4 major HBV genotypes (A, B, C and D) with a more pronounced effect on genotypes A and B isolates versus genotypes C and D isolates (after 3 days post-transfection, approximately 2 log10 copies/mL versus approximately 1 log10 copies/mL reduction in HBV DNA levels). In mice with HBV infection established by hydrodynamic injection of HBV DNA, peginterferon α-2a at a 3 µg/kg dose was active against all 4 major HBV genotypes with a more pronounced effect on genotypes A and B isolates versus genotypes C and D isolates (approximately 2 log10 copies/mL versus approximately 1 log10 copies/mL reduction in HBV DNA levels after 3 and 7 days post-infection).
Resistance
Differences in the clinical response to interferon-based therapy have been observed depending on HBV genotype. Although efficacy was demonstrated in all HBV genotypes, genotypes A and B were associated with greater efficacy responses than genotypes C and D (the lowest response being observed with genotype D). In addition, HBV gene mutations may affect interferon therapy response such as viral mutations in the precore (PC) and basal core promoter (BCP) regions.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenesis
PEGASYS did not cause DNA damage when tested in the Ames bacterial mutagenicity assay and in the in vitro chromosomal aberration assay in human lymphocytes, either in the presence or absence of metabolic activation.
Impairment of Fertility
PEGASYS may impair fertility in women. Prolonged menstrual cycles and/or amenorrhea were observed in female cynomolgus monkeys given subcutaneous injections of 600 mcg/kg/dose (7200 mcg/m2/dose) of PEGASYS every other day for one month, at approximately 180 times the recommended weekly human dose for a 60 kg person (based on body surface area). Menstrual cycle irregularities were accompanied by both a decrease and delay in the peak 17β-estradiol and progesterone levels following administration of PEGASYS to female monkeys. A return to normal menstrual rhythm followed cessation of treatment. Every other day dosing with 100 mcg/kg (1200 mcg/m2) PEGASYS (equivalent to approximately 30 times the recommended human dose) had no effects on cycle duration or reproductive hormone status.
The effects of PEGASYS on male fertility have not been studied. However, no adverse effects on fertility were observed in male Rhesus monkeys treated with non-pegylated interferon alfa-2a for 5 months at doses up to 25 × 106 IU/kg/day.
14. Clinical Studies
14.1 Chronic Hepatitis C Studies 1, 2, and 3: PEGASYS/COPEGUS Combination Therapy
Adult Patients
The safety and effectiveness of PEGASYS in combination with COPEGUS for the treatment of hepatitis C virus infection were assessed in two randomized controlled clinical trials. All subjects were adults, had compensated liver disease, detectable hepatitis C virus, liver biopsy diagnosis of chronic hepatitis, and were previously untreated with interferon. Approximately 20% of subjects in both studies had compensated cirrhosis (Child-Pugh class A). Subjects coinfected with HIV were excluded from these studies.
In Study 1, subjects were randomized to receive either PEGASYS 180 mcg subcutaneous once weekly with an oral placebo, PEGASYS 180 mcg once weekly with COPEGUS 1000 mg by mouth (body weight less than 75 kg) or 1200 mg by mouth (body weight greater than or equal to 75 kg) or Rebetron® (interferon alfa-2b 3 MIU subcutaneous three times a week plus ribavirin 1000 mg or 1200 mg by mouth). All subjects received 48 weeks of therapy followed by 24 weeks of treatment-free follow-up. COPEGUS or placebo treatment assignment was blinded. Sustained virological response was defined as undetectable (less than 50 IU/mL) HCV RNA on or after study week 68. PEGASYS in combination with COPEGUS resulted in a higher SVR compared to PEGASYS alone or interferon alfa-2b and ribavirin (Table 10). In all treatment arms, subjects with viral genotype 1, regardless of viral load, had a lower response rate.
| Interferon alfa-2b + Ribavirin 1000 mg or 1200 mg | PEGASYS + Placebo | PEGASYS + COPEGUS 1000 mg or 1200 mg | |
|---|---|---|---|
| All subjects | 197/444 (44%)* | 65/224 (29%) | 241/453 (53%)* |
| Genotype 1 | 103/285 (36%) | 29/145 (20%) | 132/298 (44%) |
| Genotypes 2-6 | 94/159 (59%) | 36/79 (46%) | 109/155 (70%) |
| |||
In Study 2 (see Table 11), all subjects received PEGASYS 180 mcg subcutaneous once weekly and were randomized to treatment for either 24 or 48 weeks and to a COPEGUS dose of either 800 mg or 1000 mg/1200 mg (for body weight less than 75 kg/greater than or equal to 75 kg). Assignment to the four treatment arms was stratified by viral genotype and baseline HCV viral titer. Subjects with genotype 1 and high viral titer (defined as greater than 2 × 106 HCV RNA copies/mL serum) were preferentially assigned to treatment for 48 weeks.
HCV Genotypes
HCV 1 and 4 – Irrespective of baseline viral titer, treatment for 48 weeks with PEGASYS and 1000 mg or 1200 mg of COPEGUS resulted in higher SVR (defined as undetectable HCV RNA at the end of the 24-week treatment-free follow-up period) compared to shorter treatment (24 weeks) and/or 800 mg COPEGUS.
HCV 2 and 3 – Irrespective of baseline viral titer, treatment for 24 weeks with PEGASYS and 800 mg of COPEGUS resulted in a similar SVR compared to longer treatment (48 weeks) and/or 1000 mg or 1200 mg of COPEGUS (see Table 11).
The numbers of subjects with genotype 5 and 6 were too few to allow meaningful assessment.
| 24 Weeks Treatment | 48 Weeks Treatment | |||||
|---|---|---|---|---|---|---|
| PEGASYS + COPEGUS 800 mg (N=207) | PEGASYS + COPEGUS 1000 mg or 1200 mg*
(N=280) | PEGASYS + COPEGUS 800 mg (N=361) | PEGASYS + COPEGUS 1000 mg or 1200 mg*
(N=436) | |||
| Genotype 1 | 29/101 (29%) | 48/118 (41%) | 99/250 (40%) | 138/271 (51%) | ||
| Genotypes 2, 3 | 79/96 (82%) | 116/144 (81%) | 75/99 (76%) | 117/153 (76%) | ||
| Genotype 4 | 0/5 (0%) | 7/12 (58%) | 5/8 (63%) | 9/11 (82%) | ||
| ||||||
Other Treatment Response Predictors
Treatment response rates are lower in subjects with poor prognostic factors receiving pegylated interferon alpha therapy. In studies 1 and 2, treatment response rates were lower in subjects older than 40 years (50% vs. 66%), in subjects with cirrhosis (47% vs. 59%), in subjects weighing over 85 kg (49% vs. 60%), and in subjects with genotype 1 with high vs. low viral load (43% vs. 56%). African-American subjects had lower response rates compared to Caucasians.
Paired liver biopsies were performed on approximately 20% of subjects in studies 4 and 5. Modest reductions in inflammation compared to baseline were seen in all treatment groups.
In studies 1 and 2, lack of early virologic response by 12 weeks (defined as HCV RNA undetectable or greater than 2 log10 lower than baseline) was grounds for discontinuation of treatment. Of subjects who lacked an early viral response by 12 weeks and completed a recommended course of therapy despite a protocol-defined option to discontinue therapy, 5/39 (13%) achieved an SVR. Of subjects who lacked an early viral response by 24 weeks, 19 completed a full course of therapy and none achieved an SVR.
Pediatric Patients
Previously untreated pediatric subjects 5 through 17 years of age (55% less than 12 years old) with CHC, compensated liver disease and detectable HCV RNA were treated with COPEGUS approximately 15 mg/kg/day plus PEGASYS 180 mcg/1.73 m2 × body surface area once weekly for 48 weeks. All subjects were followed for 24 weeks post-treatment. Sustained virological response (SVR) was defined as undetectable (less than 50 IU/mL) HCV RNA on or after study week 68. A total of 114 subjects were randomized to receive either combination treatment of COPEGUS plus PEGASYS or PEGASYS monotherapy; subjects failing PEGASYS monotherapy at 24 weeks or later could receive open-label COPEGUS plus PEGASYS. The initial randomized arms were balanced for demographic factors; 55 subjects received initial combination treatment of COPEGUS plus PEGASYS and 59 received PEGASYS plus placebo; in the overall intent-to-treat population, 45% were female, 80% were Caucasian, and 81% were infected with HCV genotype 1. The SVR results are summarized in Table 12.
| PEGASYS 180 mcg/1.73 m2 × BSA + COPEGUS 15 mg/kg (N=55)* | PEGASYS 180 mcg/1.73 m2 × BSA + Placebo*
(N=59) | |
|---|---|---|
| All HCV genotypes† | 29 (53%) | 12 (20%) |
| HCV genotype 1 | 21/45 (47%) | 8/47 (17%) |
| HCV non-genotype 1‡ | 8/10 (80%) | 4/12 (33%) |
| ||
14.2 Chronic Hepatitis C and Coinfection with HIV (CHC/HIV) Study 4: PEGASYS Monotherapy and PEGASYS/COPEGUS Combination Therapy
In Study 4, subjects with CHC/HIV were randomized to receive either PEGASYS 180 mcg subcutaneous once weekly plus an oral placebo, PEGASYS 180 mcg once weekly plus COPEGUS 800 mg by mouth daily or ROFERON-A (interferon alfa-2a), 3 MIU subcutaneous three times a week plus COPEGUS 800 mg by mouth daily. All subjects received 48 weeks of therapy and sustained virologic response (SVR) was assessed at 24 weeks of treatment-free follow-up. COPEGUS or placebo treatment assignment was blinded in the PEGASYS treatment arms. All subjects were adults, had compensated liver disease, detectable hepatitis C virus, liver biopsy diagnosis of chronic hepatitis C, and were previously untreated with interferon. Subjects also had CD4+ cell count greater than or equal to 200 cells/mm3 or CD4+ cell count greater than or equal to 100 cells/mm3 but less than 200 cells/mm3 and HIV-1 RNA less than 5,000 cells/mm3, and stable status of HIV. Approximately 15% of subjects in the study had cirrhosis. Results are shown in Table 13 .
| ROFERON-A + COPEGUS 800 mg (N=289) | PEGASYS + Placebo (N=289) | PEGASYS + COPEGUS 800 mg (N=290) | |
|---|---|---|---|
| All subjects | 33 (11%) | 58 (20%) | 116 (40%) |
| Genotype 1 | 12/171 (7%) | 24/175 (14%) | 51/176 (29%) |
| Genotypes 2, 3 | 18/89 (20%) | 32/90 (36%) | 59/95 (62%) |
Treatment response rates are lower in CHC/HIV subjects with poor prognostic factors (including HCV genotype 1, HCV RNA greater than 800,000 IU/mL, and cirrhosis) receiving pegylated interferon alpha therapy. Geographic region is not a prognostic factor for response. However, poor prognostic factors occur more frequently in the US population than in the non-US population.
Of the subjects who did not demonstrate either undetectable HCV RNA or at least a 2 log10 reduction from baseline in HCV RNA titer by 12 weeks of PEGASYS and ribavirin combination therapy, 2% (2/85) achieved an SVR.
In CHC subjects with HIV coinfection who received 48 weeks of PEGASYS alone or in combination with ribavirin treatment, mean and median HIV RNA titers did not increase above baseline during treatment or 24 weeks post-treatment.
14.3 Chronic Hepatitis C Studies 5, 6, and 7: PEGASYS Monotherapy
The safety and effectiveness of PEGASYS for the treatment of hepatitis C virus infection were assessed in three randomized, open-label, active-controlled clinical studies. All subjects were adults, had compensated liver disease, detectable hepatitis C virus (HCV), liver biopsy diagnosis of chronic hepatitis, and were previously untreated with interferon. All subjects received therapy by subcutaneous injection for 48 weeks, and were followed for an additional 24 weeks to assess the durability of response. In studies 5 and 6, approximately 20% of subjects had cirrhosis or bridging fibrosis. Study 7 enrolled subjects with a histological diagnosis of cirrhosis (78%) or bridging fibrosis (22%).
In Study 5 (n=630), subjects received either ROFERON-A (interferon alfa-2a) 3 MIU three times a week, PEGASYS 135 mcg once weekly or PEGASYS 180 mcg once weekly. In Study 6 (n=526), subjects received either ROFERON-A 6 MIU three times a week for 12 weeks followed by 3 MIU three times a week for 36 weeks or PEGASYS 180 mcg once weekly. In Study 7 (n=269), subjects received ROFERON-A 3 MIU three times a week, PEGASYS 90 mcg once weekly or PEGASYS 180 mcg once each week.
In all three studies, treatment with PEGASYS 180 mcg resulted in significantly more subjects who experienced a sustained response (defined as undetectable HCV RNA [less than 50 IU/mL] using the COBAS AMPLICOR® HCV Test, version 2 and normalization of ALT on or after study week 68) compared to treatment with ROFERON-A.
In Study 5, response to PEGASYS 135 mcg was not different from response to 180 mcg. In Study 7, response to PEGASYS 90 mcg was intermediate between PEGASYS 180 mcg and ROFERON-A.
| Study 5 | Study 6 | Study 7 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Roferon-A 3 MIU (N=207) | PEGASYS 180 mcg (N=208) | Diff*
(95% CI) | Roferon-A 6/3 MIU† (N=261) | PEGASYS 180 mcg (N=265) | Diff*
(95% CI) | Roferon-A 3 MIU (N=86) | PEGASYS 180 mcg (N=87) | Diff*
(95% CI) | |||||
| Combined Virologic and Biologic Sustained Response‡ | 11% | 24% | 13 (6, 20) | 17% | 35% | 18 (11, 25) | 7% | 23% | 16 (6, 26) | ||||
| Sustained Virologic Response | 11% | 26% | 15 (8, 23) | 19% | 38% | 19 (11, 26) | 8% | 30% | 22 (11, 33) | ||||
| |||||||||||||
Matched pre- and post-treatment liver biopsies were obtained in approximately 70% of subjects. Similar modest reductions in inflammation compared to baseline were observed in all treatment groups.
Of the subjects who did not demonstrate either undetectable HCV RNA or at least a 2 log10 drop in HCV RNA titer from baseline by 12 weeks of PEGASYS 180 mcg therapy, 2% (3/156) achieved a sustained virologic response [see Dosage and Administration (2.2)].
Averaged over Study 5, Study 6, and Study 7, response rates to PEGASYS were 23% among subjects with viral genotype 1 and 48% among subjects with other viral genotypes. The treatment response rates were similar in men and women.
14.4 Chronic Hepatitis B Studies 8, 9 and 10: PEGASYS Monotherapy
Adult Patients
The safety and effectiveness of PEGASYS for the treatment of CHB were assessed in controlled clinical trials in HBeAg-positive (Study 8) and HBeAg-negative (Study 9) subjects with CHB.
Subjects were randomized to PEGASYS 180 mcg subcutaneous once weekly, PEGASYS 180 mcg subcutaneous once weekly combined with lamivudine 100 mg once daily by mouth or lamivudine 100 mg once daily by mouth. All subjects received 48 weeks of their assigned therapy followed by 24 weeks of treatment-free follow-up. Assignment to receipt of PEGASYS or no PEGASYS was not masked.
All subjects were adults with compensated liver disease, had chronic hepatitis B virus (HBV) infection, and evidence of HBV replication (serum HBV greater than 500,000 copies/mL for Study 8 and greater than 100,000 copies/mL for Study 9) as measured by PCR (COBAS AMPLICOR® HBV Assay). All subjects had serum alanine aminotransferase (ALT) between 1 and 10 times the upper limit of normal (ULN) and liver biopsy findings compatible with the diagnosis of chronic hepatitis.
The results observed in the PEGASYS and lamivudine monotherapy groups are shown in Table 15.
| Study 8 HBeAg positive | Study 9 HBeAg negative | |||||
|---|---|---|---|---|---|---|
| LamivudineN = 272 | PEGASYSN = 271 | LamivudineN = 181 | PEGASYSN = 177 | |||
| EOT* | EOF† | EOF† | EOT* | EOF† | EOF† | |
| HBeAg Seroconversion (%) | 20 | 19 | 32 | NA | NA | NA |
| HBV DNA Response (%)‡ | 62 | 22 | 32 | 85 | 29 | 43 |
| ALT Normalization (%) | 62 | 28 | 41 | 73 | 44 | 59 |
| HBsAg Seroconversion (%) | 0 | 0 | 3 | 1 | 0 | 3 |
| N = 184 | N = 207 | N = 125 | N = 143 | |||
| Histological Improvement (%)§ | ND | 40 | 41 | ND | 41 | 48 |
| Changes in Ishak fibrosis score compared to baseline (%): | ||||||
| - Improved¶ | ND | 32 | 25 | ND | 31 | 32 |
| - Unchanged | 20 | 25 | 23 | 30 | ||
| - Worsened¶ | 16 | 26 | 15 | 19 | ||
| ||||||
PEGASYS co-administered with lamivudine did not result in any additional sustained response when compared to PEGASYS monotherapy.
Conclusions regarding comparative efficacy of PEGASYS and lamivudine treatment based upon the end of follow-up results are limited by the different mechanisms of action of the two compounds. Most treatment effects of lamivudine are unlikely to persist 24 weeks after therapy is withdrawn.
Pediatric Patients
Study 10 was conducted in previously untreated pediatric subjects aged 3 to less than 18 years (51% < 12 years old) with HBeAg-positive CHB in the immune-active phase. Subjects with cirrhosis were not enrolled in this study. A total of 151 subjects without advanced fibrosis were randomized 2:1 to PEGASYS (group A, n=101) or untreated control (group B, n=50), respectively. Subjects with advanced fibrosis were assigned to PEGASYS treatment (group C, n=10). Subjects in groups A and C (n=111) were treated with PEGASYS once weekly for 48 weeks according to dosing regimen based on body surface area, whereas subjects in group B were observed for a period of 48 weeks (principal observation period). Subjects in group B had the choice to switch to treatment with PEGASYS after Week 48 of the principal observation period. All subjects were followed up for 24 weeks post-treatment (groups A and C), or post-principal observation period (group B). Response rates in groups A and B at the end of 24 weeks follow-up are presented in Table 16. Efficacy response in group C to PEGASYS treatment was similar to that seen in group A.
| Endpoints* | Group A (PEGASYS treatment) (N=101) | Group B (Untreated)†
(N=50) |
|---|---|---|
| HBeAg Seroconversion | 26%‡ | 6.0% |
| HBV DNA < 20,000 IU/mL§ | 34% | 4.0% |
| HBV DNA < 2,000 IU/mL | 29% | 2.0% |
| ALT Normalization | 52% | 12.0% |
| HBsAg Seroconversion | 8% | 0.0% |
| Loss of HBsAg | 9% | 0.0% |
| ||
16. How Supplied/Storage and Handling
PEGASYS (peginterferon alfa-2a) injection is a sterile, preservative-free, colorless to slightly yellowish solution administered subcutaneously.
| Each PEGASYS Single-Dose Vial Package Contains: | |
| A box containing 180 mcg per 1 mL solution in a single-dose vial. | (NDC 0004-0350-09) |
| Each PEGASYS Prefilled Syringe Monthly Convenience Pack Contains: | |
| A box containing four 180 mcg per 0.5 mL (½ cc) single-dose prefilled syringes with 4 needles. Each prefilled syringe is supplied with a 27-gauge, ½-inch needle with a needle-stick protection device. | (NDC 0004-0357-30) |
Storage and Handling
Store in the refrigerator at 2°C to 8°C (36°F to 46°F). Do not leave PEGASYS out of the refrigerator for more than 24 hours. Do not freeze or shake. Protect from light. Vials and prefilled syringes are for single use only. Discard any unused portion remaining in the vial, prefilled syringe.
Disposal Instructions
If home use is prescribed, a puncture-resistant container for the disposal of used needles and syringes should be supplied to the patients. Patients should be thoroughly instructed in the importance of proper disposal and cautioned against any reuse of any needles and syringes. The full container should be disposed of according to the directions provided by the physician [see FDA-Approved Medication Guide].
17. Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use)
Patients receiving PEGASYS alone or in combination with an approved HCV antiviral drug should be directed in its appropriate use, informed of the benefits and risks associated with treatment, and referred to FDA-approved patient labeling (Medication Guide and Instructions for Use).
Pregnancy
Patients must be informed that ribavirin must not be used by women who are pregnant or by men whose female partners are pregnant. Ribavirin therapy should not be initiated until a report of a negative pregnancy test has been obtained immediately before starting therapy. Female patients of childbearing potential and male patients with female partners of childbearing potential must be advised of the teratogenic/embryocidal risks and must be instructed to practice effective contraception during ribavirin therapy and for 6 months post-therapy. Patients should be advised to notify the healthcare provider immediately in the event of a pregnancy [see Contraindications (4) and Warnings and Precautions (5.1)].
Women of childbearing potential and men must use two forms of effective contraception during treatment and during the 6 months after treatment has been stopped; routine monthly pregnancy tests must be performed during this time [see Contraindications (4), Warnings and Precautions (5.1), Use in Specific Populations (8.1), and ribavirin prescribing information].
To monitor maternal and fetal outcomes of pregnant women exposed to ribavirin, the Ribavirin Pregnancy Registry has been established. Patients should be encouraged to register by calling 1-800-593-2214.
Flu-like symptoms
Inform patients that flu-like symptoms are very common in patients taking PEGASYS. Symptoms may include tiredness, weakness, fever, chills, muscle aches, joint pain, and headaches. Inform patients that some of these symptoms may be decreased by injecting PEGASYS in the evening. Also inform patients which over-the-counter medicines can be taken to help prevent or decrease some of the symptoms.
Laboratory Evaluations and Hydration
Patients should be advised that laboratory evaluations are required before starting therapy and periodically thereafter [see Warnings and Precautions (5.17)]. Patients should be instructed to remain well hydrated, especially during the initial stages of treatment.
General Information
Patients should be questioned about prior history of drug abuse before initiating PEGASYS; as relapse of drug addiction and drug overdoses have been reported in patients treated with interferons.
Patients should be informed that it is not known if therapy with PEGASYS will prevent transmission of HBV infection to others or prevent cirrhosis, liver failure or liver cancer that might result from HBV infection.
Patients should be informed that the effect of treatment of hepatitis C infection on transmission is not known, and that appropriate precautions to prevent transmission of the hepatitis C virus during treatment or in the event of treatment failure should be taken.
Patients who develop dizziness, confusion, somnolence, and fatigue should be cautioned to avoid driving or operating machinery.
Patients should be advised to avoid drinking alcohol to reduce the chance of further injury to the liver.
Patients should not switch to another brand of interferon without consulting their healthcare provider.
Dosing Instructions
Patients should be advised to take their prescribed dose of PEGASYS on the same day and approximately same time each week. Patients should also be advised that if they miss a dose, but remember within 2 days, to take their missed dose as soon as they remember and then to take their next dose on the day they normally do. If they remember when more than 2 days have passed, patients should be advised to consult their healthcare provider. Patients should also be advised to consult their healthcare provider if the full dose is not received (e.g., leakage around the injection site).
Patients must be instructed on the use of aseptic techniques when administering PEGASYS. Appropriate training for preparation using the vial and prefilled syringe must be given by a healthcare provider, including a careful review of the PEGASYS Medication Guide and Instructions for Use for the vial and prefilled syringe.
Patients should be instructed to allow the vial and prefilled syringe to come to room temperature and for condensation on the outside of the prefilled syringe to disappear before use. The following instructions should be given:
- Vial: warm the refrigerated medicine by gently rolling in the palms of the hands for about one minute.
- Pre-filled syringe: lay the syringe on a flat clean surface and wait a few minutes until it reaches room temperature. If condensation water is observed on the outside of the syringe, wait another few minutes until it disappears.
Patients should be advised not to shake the vial and prefilled syringe as foaming may occur.
Patients should be advised to choose a different place on either the thigh or abdomen each time an injection is made.
Spl Unclassified Section
PEGASYS® is a registered trademark of Hoffmann-La Roche Inc.
Manufactured by: Hoffmann-La Roche, Inc.c/o Genentech, Inc. A Member of the Roche Group1 DNA WaySouth San Francisco, CA 94080-4990U.S. License No. 0136
Distributed by: Genentech USA, Inc. A Member of the Roche Group1 DNA WaySouth San Francisco, CA 94080-4990
© 2020 Genentech, Inc. All rights reserved.
Medication Guide
| This Medication Guide has been approved by the U.S. Food and Drug Administration | Revised: October 2020 | |
Medication GuidePEGASYS® (PEG-ah-sis)(peginterferon alfa-2a)Injectionfor Subcutaneous Use | ||
Important: If you are taking PEGASYS with other medicines for treatment of hepatitis C, you should also read the Medication Guides or Patient Information that comes with the other medicines. | ||
What is the most important information I should know about PEGASYS? Ribavirin in combination with PEGASYS may cause birth defects or death of your unborn baby. If you are pregnant or your sexual partner is pregnant or plans to become pregnant, do not take PEGASYS and Ribavirin combination therapy. You or your sexual partner should not become pregnant during treatment with PEGASYS and Ribavirin combination therapy and for 6 months after treatment is over. You must use 2 effective forms of birth control, one of which should be a condom with spermicide during treatment with PEGASYS and Ribavirin combination therapy, and for the 6 months after you stop treatment. | ||
| ||
Mental health problems and suicide. PEGASYS may cause you to develop mood or behavioral problems, including: | ||
| ||
| Heart problems. PEGASYS may cause heart problems, including: | ||
|
| |
Stroke or symptoms of a stroke. Symptoms may include weakness, loss of coordination, and numbness. Stroke or symptoms of a stroke may happen in people who have some risk factors or no known risk factors for a stroke. New or worsening autoimmune problems. Some people taking PEGASYS develop autoimmune problems (a condition where the body's immune cells attack other cells or organs in the body), such as rheumatoid arthritis, systemic lupus erythematosus, and psoriasis. In some people who already have an autoimmune problem, it may get worse during your treatment with PEGASYS. Infections. Symptoms may include: | ||
|
| |
Call your healthcare provider right away if you get any of these symptoms during treatment with PEGASYS: Before and during treatment with PEGASYS you will need to see your healthcare provider regularly and have blood tests to make sure that your treatment is working and to check for side effects. PEGASYS can cause serious side effects. Some of these side effects may cause death. Tell your healthcare provider right away if you have any of these symptoms during treatment with PEGASYS. For more information about side effects, see "What are the possible side effects of PEGASYS?" | ||
What is PEGASYS? PEGASYS is a prescription medicine that is:
PEGASYS should not be used alone or with Ribavirin, without taking other HCV antiviral medicines, to treat people with CHC who have taken interferon-alfa and it did not work. PEGASYS should not be used to treat people with CHC who have received an organ transplant. PEGASYS is a prescription medicine that is:
It is not known if PEGASYS is safe and effective in:
| ||
Who should not take PEGASYS? Do not take PEGASYS if you:
Do not give PEGASYS to a baby under 1 year of age. PEGASYS contains benzyl alcohol. Benzyl alcohol can cause nervous system problems and other problems which may lead to death. Do not take PEGASYS in combination with Ribavirin if you:
Talk to your healthcare provider before taking PEGASYS if you have any of these conditions. | ||
Before taking PEGASYS tell your healthcare provider about all of your medical conditions, including if you :
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. PEGASYS and certain other medicines may affect each other and cause side effects. Especially tell your healthcare provider if you take: | ||
| ||
| ||
How should I take PEGASYS?
| ||
What should I avoid while taking PEGASYS?
| ||
What are the possible side effects of PEGASYS? PEGASYS can cause serious side effects including:
| ||
Symptoms of low blood sugar may include:
|
| |
Symptoms of high blood sugar or diabetes may include:
|
| |
| ||
|
| |
| ||
|
| |
You may need to have a chest X-ray or other tests if you develop fever, cough, shortness of breath or other symptoms of a lung problem during treatment with PEGASYS. | ||
| ||
|
| |
| ||
|
| |
| ||
|
| |
Tell your healthcare provider right away if you have any of the symptoms listed above. The most common side effects of PEGASYS include:
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the side effects of PEGASYS. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Genentech at 1-888-835-2555. | ||
How should I store PEGASYS?
Keep PEGASYS and all medicines out of the reach of children. | ||
General information about the safe and effective use of PEGASYS. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use PEGASYS for a condition for which it was not prescribed. Do not give PEGASYS to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information that is written for health professionals. | ||
What are the ingredients in PEGASYS? Active ingredient: interferon alfa-2a Inactive ingredients: acetic acid, benzyl alcohol, polysorbate 80, sodium acetate trihydrate, and sodium chloride. Manufactured by: Hoffmann-La Roche, Inc. c/o Genentech, Inc. A Member of the Roche Group 1 DNA Way South San Francisco, CA 94080-4990 U.S. License No. 0136PEGASYS® is a registered trademark of Hoffmann-La Roche Inc. | ||
Instructions For Use Section
First read the Medication Guide that comes with PEGASYS for the most important information you need to know about PEGASYS. Be sure that you read, understand and follow these Instructions for Use before injecting PEGASYS. Your healthcare provider should show you how to prepare, measure and inject PEGASYS properly before you use it for the first time. Ask your healthcare provider if you have any questions.
PEGASYS prefilled syringes come in a Monthly Convenience Pack that contains 4 prefilled syringes of PEGASYS in a box with 4 needles. Each needle has a needle-stick protection device.
Before starting, collect all of the supplies that you will need to use for preparing and injecting PEGASYS. You will need the following supplies:
- 1 single-dose disposable prefilled syringe of PEGASYS
- 1 needle with needle-stick protection device
- several alcohol pads
- You will also need a puncture-resistant disposable container to throw away used prefilled syringes and needles as soon as you finish your injection. See "How should I dispose of used syringes and needles?"
Important:
- Never reuse disposable prefilled syringes and needles.
- Throw away the prefilled syringe of PEGASYS after you use it 1 time, even if there is any medicine left in it.
- Do not shake PEGASYS. If shaken, PEGASYS may not work properly.
How should I prepare a dose of PEGASYS?
- Find a well-lit, clean, flat surface such as a table.
- Take a carton containing PEGASYS out of the refrigerator. Check the date on the carton the PEGASYS comes in. Make sure the expiration date has not passed. Do not use if the expiration date has passed (see Figure A).
Figure A:
- Remove the prefilled syringe of PEGASYS from the carton. Look at the prefilled syringe of PEGASYS. The solution should be clear and colorless to slightly yellowish, without particles (see Figure B). If there is foam in the solution, put it back in the refrigerator for use at a later time and use another syringe.
Figure B:
Do not use the prefilled syringe of PEGASYS if:
- the medicine remains cloudy after a few minutes at room temperature
- has particles
- the medicine is not colorless to slightly yellowish
- the expiration date has passed (see Figure C).
Figure C:
- Wash your hands well with soap and warm water. Keep your work area, your hands, and injection site clean to decrease the risk of infection.
- Lay the syringe on a flat clean surface and wait a few minutes until it reaches room temperature. If you notice condensation water on the outside of the syringe, wait another few minutes until it disappears.
How do I attach the needle to the PEGASYS prefilled syringe?
- Remove the needle from its package. Do not remove the needle shield yet. Keep the needle covered until just before you give the injection (see Figure D).
Figure D:
- Remove and throw away the rubber cap from the tip of the syringe barrel (see Figure E).
Figure E:
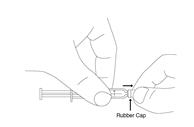
- With one hand, hold the syringe by the barrel. With your other hand, hold the needle close to the hub where the green needle cover connects to the syringe (see Figure F).
Figure F:
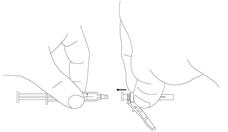
- Push the needle onto the syringe and tighten by using an easy twisting motion in the direction of the arrow (see Figure G).
Figure G:
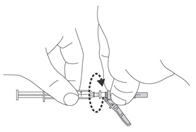
Here is a picture of what the syringe will look like after you finish attaching the needle (see Figure H).
Figure H:
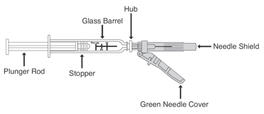
- Lay the syringe and needle down on your clean work surface. Be sure that the plastic needle shield covers the needle. Never let the needle touch any surface.
How should I choose a site for injection?
- You can inject PEGASYS under the skin on your stomach or thigh (see Figure I). Avoid your navel and waistline. You should use a different place each time you give yourself an injection.
Figure I:
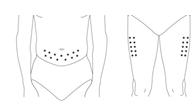
- Clean the area using an alcohol pad. Let the skin air dry.
How do I prepare the PEGASYS prefilled syringe for injection?
- Pull the green needle cover back from the needle toward the syringe barrel. The green needle cover will stay in the position you set. Do not remove it. This is the needle-stick protection device (see Figure J).
Figure J:
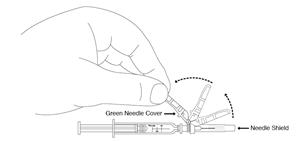
- Hold the syringe and needle tightly at the hub. Gently rock the plastic needle shield back and forth to prepare for removal. Remove the plastic needle shield by pulling it straight off (see Figure K).
Figure K:
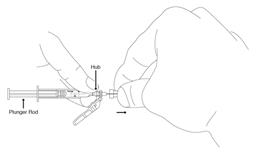
- Remove air bubbles from the syringe.
- Hold the syringe with the needle pointing up to the ceiling.
- Using your thumb and finger, gently tap the syringe to bring air bubbles to the top (see Figure L).
- Press the plunger in slightly to push air bubbles out of the syringe.
Figure L:
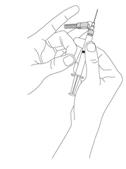
- Depending on the dose of PEGASYS that your healthcare provider prescribes, you may have to get rid of (discard) some of the medicine from the prefilled syringe before you inject the medicine. The syringe has markings for 180 mcg, 135 mcg, and 90 mcg. Your healthcare provider will tell you which mark to use (see Figure M and Figure N).
Figure M:
Figure N:
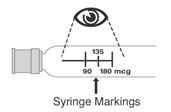
Do not decrease or increase your dose of PEGASYS unless your healthcare provider tells you to.
How do I give the injection of PEGASYS?
- Position the point of the needle (the bevel) so it is facing up (see Figure O).
Figure O:
- Pinch a fold of skin on your stomach or thigh firmly with your thumb and forefinger (see Figure P).
Figure P:
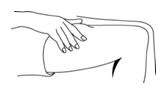
- Hold the syringe like a pencil at a 45° to 90° angle to your skin. With a quick "dart-like" motion, push the needle into the skin as far as it will go (see Figure Q).
Figure Q:
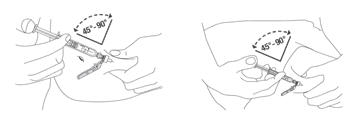
- After the needle is inserted, remove the hand used to pinch the skin and use it to hold the syringe barrel.
- Pull the plunger of the syringe back slightly.
-
If blood comes into the syringe, the needle has entered a blood vessel.
- Do not inject PEGASYS. Withdraw the needle and throw away the syringe and needle in the puncture-resistant container. See "How should I dispose of used syringes and needles?"
- Then, repeat steps 1 through 16 with a new prefilled syringe and prepare a new injection site.
- If no blood is present in the syringe, inject the medicine by gently pressing the plunger all the way down the syringe barrel, until the syringe is empty.
- When the syringe is empty, pull the needle out of the skin. Wipe the area with an alcohol pad.
- To prevent needle-stick injuries, before you dispose of the syringe and needle, push the green needle cover toward the needle (see Figure R). Then place the free end of the green cap on a flat surface and push down with a firm and quick motion until it clicks and covers over the needle (see Figure S).
Figure R:
Figure S:
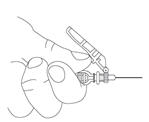
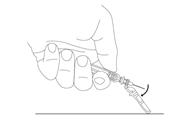
- Throw away the used syringe and needle right away as described below. See "How should I dispose of used syringes and needles?"
How should I dispose of used syringes and needles?
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal .
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Always keep the puncture-resistant container out of the reach of children.
How should I store PEGASYS?
- Store PEGASYS prefilled syringes in a refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not leave PEGASYS out of the refrigerator for more than 24 hours.
- Do not freeze or shake PEGASYS.
- Protect PEGASYS from light.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: October 2017
PEGASYS® is a registered trademark of Hoffmann-La Roche Inc.
Manufactured by: Hoffmann-La Roche, Inc.c/o Genentech, Inc. A Member of the Roche Group1 DNA WaySouth San Francisco, CA 94080-4990U.S. License No. 0136
Distributed by: Genentech USA, Inc. A Member of the Roche Group1 DNA WaySouth San Francisco, CA 94080-4990
© 2017 Genentech, Inc. All rights reserved.
Instructions For Use Section
First read the Medication Guide that comes with PEGASYS for the most important information you need to know about PEGASYS. Be sure that you read, understand and follow these Instructions for Use before injecting PEGASYS. Your healthcare provider should show you how to prepare, measure, and inject PEGASYS properly before you use it for the first time. Ask your healthcare provider if you have any questions.
Before starting, collect all of the supplies that you will need to use for preparing and injecting PEGASYS. You will need the following supplies:
- 1 vial of PEGASYS
- 1 single-use disposable syringe and needle
- several alcohol pads
- You will also need a puncture-resistant disposable container to throw away used syringes, needles, and vials as soon as you finish your injection. See "How should I dispose of used syringes, needles, and vials?"
Follow your healthcare provider's instructions for the type of syringe and needle to use to prepare and inject your dose. If you will be injecting a child with PEGASYS, you will need a special syringe called a tuberculin syringe, which can measure doses of PEGASYS that are 1 milliliter (1mL) or less. When you get your prescription from the pharmacy, ask your pharmacist for the syringe and needle that you need to prepare and inject a dose of PEGASYS from the single-dose vial.
Important:
- Never reuse disposable syringes and needles.
- Throw away the vial of PEGASYS after you use it 1 time even if there is medicine left in the vial.
- Do not shake PEGASYS. If shaken, PEGASYS may not work properly.
How should I prepare a dose of PEGASYS?
- Find a well-lit, clean, flat working surface such as a table.
- Take a carton containing PEGASYS out of the refrigerator. Check the date on the carton the PEGASYS comes in. Make sure the expiration date has not passed. Do not use if the expiration date has passed (see Figure A).
Figure A:
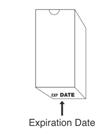
- Wash your hands well with soap and warm water. Keep your work area, your hands, and injection site clean to decrease the risk of infection.
- Remove the vial of PEGASYS from the carton. Look at the vial of PEGASYS. The solution should be clear and colorless to slightly yellowish, without particles (see Figure B).
Figure B:
Do not use the vial of PEGASYS if:
- the medicine is cloudy
- has particles
- the medicine is not colorless to slightly yellowish
- the expiration date has passed (see Figure B)
- Warm the refrigerated medicine by gently rolling it in the palms of your hands for about one minute. Do not shake PEGASYS.
- Remove (flip off) the plastic cap from the top of the PEGASYS vial (see Figure C). Clean the rubber stopper on the top of the vial with an alcohol pad (see Figure D).
If you are not sure how much medicine to use or which mark on the syringe to use, stop and call your healthcare provider right away.Figure C:
Figure D:
- Open the package for the syringe you are using and if it does not have a needle attached, then attach a new needle to the syringe.
- Remove the protective cap from the needle on the syringe. Never let the needle touch any surface. Fill the syringe with air by pulling back on the plunger to the mark on the syringe barrel that matches the dose prescribed by your healthcare provider (see Figure E).
Figure E:
- Hold the vial of PEGASYS on your flat surface. Do not touch the cleaned rubber stopper.
- Push the needle straight down through the middle of the rubber stopper on the vial. Slowly inject all the air from the syringe into the air space above the solution. Do not inject air into the fluid (see Figure F).
Figure F:
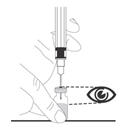
- Keep the needle in the vial. Turn the vial upside down.Make sure the tip of the needle is in the PEGASYS solution.Slowly pull the plunger back to fill the syringe with PEGASYS solution to the dose (mL or cc markings on the syringe) that matches the dose prescribed by your healthcare provider (see Figure G).
Figure G:
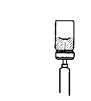
- Do not remove the needle from the vial. Lay the vial and syringe on its side on your flat work surface until you are ready to inject the PEGASYS solution (see Figure H).
Figure H:
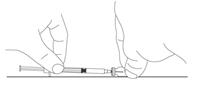
How should I choose a site for injection?
- You can inject PEGASYS under the skin on your stomach or thigh (see Figure I). Avoid your navel and waistline. You should use a different place each time you give yourself an injection.
Figure I:
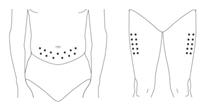
- Clean the area using an alcohol pad and let the skin air dry.
How should I give an injection?
- Pick up the vial and syringe from your flat work surface. Remove the syringe and needle from the vial.
- Hold the syringe in the hand that you will use to inject PEGASYS.
- Do not touch the needle or allow it to touch the work surface.
- Remove air bubbles from the syringe.
- Hold the syringe with the needle pointing up to the ceiling.
- Using your thumb and finger, tap the syringe to bring air bubbles to the top (see Figure J).
- Press the plunger in slightly to push air bubbles out of the syringe.
Figure J:
- Position the point of the needle (the bevel) so it is facing up (see Figure K).
Figure K:
- Pinch a fold of skin on your stomach or thigh firmly between your thumb and forefinger (see Figure L).
Figure L:
- Hold the syringe like a pencil at a 45° to 90° angle to your skin. With a quick "dart-like" motion, push the needle into the skin as far as it will go (see Figure M).
Figure M:
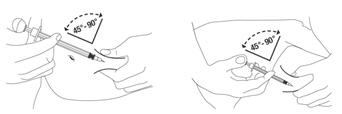
- After the needle is inserted, remove the hand used to pinch the skin and use it to hold the syringe barrel.
- Pull the plunger of the syringe back slightly.
-
If blood comes into the syringe, the needle has entered a blood vessel.
- Do not inject PEGASYS. Withdraw the needle and throw away the syringe, needle, and vial in the puncture-resistant container. See "How should I dispose of used syringes, needles, and vials?"
- Then, repeat steps 1 through 19 with a new vial of PEGASYS and inject the medicine at a new injection site.
- If no blood is present in the syringe, inject the medicine by gently pressing the plunger all the way down the syringe barrel, until the syringe is empty.
- When the syringe is empty, pull the needle out of the skin. Wipe the area with an alcohol pad.
- Throw away the used syringe, needle, and vial. See "How should I dispose of used syringes, needles, and vials?"
How should I dispose of used syringes, needles, and vials?
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal .
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store PEGASYS?
- Store PEGASYS single-dose vials in a refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not leave PEGASYS out of the refrigerator for more than 24 hours.
- Do not freeze or shake PEGASYS.
- Protect PEGASYS from light.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: October 2017
PEGASYS® is a registered trademark of Hoffmann-La Roche Inc.
Manufactured by: Hoffmann-La Roche, Inc.c/o Genentech, Inc. A Member of the Roche Group1 DNA WaySouth San Francisco, CA 94080-4990U.S. License No. 0136
Distributed by: Genentech USA, Inc. A Member of the Roche Group1 DNA WaySouth San Francisco, CA 94080-4990
© 2017 Genentech, Inc. All rights reserved.
Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
Package Label. Principal Display Panel
Package Label. Principal Display Panel
NDC 0004-0357-30
ATTENTION PHARMACIST:Each patient is required to receive the enclosed Medication Guide.
Refrigerate Immediately
Pegasys® (peginterferon alfa-2a)
Rx only
180 mcg/0.5 mL
For Subcutaneous Injection Only Sterile Prefilled Syringes Monthly Convenience Pack Package Contains:4 Single-Use Prefilled Syringes Pegasys® 180 mcg/0.5 mL, NDC 0004-0352-30 4 Needles (27-gauge, 1/2-inch)
Each Prefilled Syringe Contains: 180 mcg/0.5 mL
Genentech
10174119

