Chronic hepatitis B virus (HBV) infection is a dynamic disease process that can transition through various phases depending on the interplay between the virus and the host immune response (Figure 1).[1,2,3] Ultimately, it is the ongoing cycles of host-mediated immune response to the virus and associated inflammatory activity, or flares, that are thought to drive hepatocyte injury. Each cycle of chronic necroinflammation and inefficient clearance of the virus can contribute to the development of liver fibrosis and cumulative risk of cirrhosis.[2] Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and HBV DNA levels are, therefore, the cornerstone of HBV disease monitoring, as they provide a window into this dysfunctional interaction between host and virus. Regularly assessing these biomarkers over time provides us with the most readily available guide to deciding when antiviral therapy is indicated. If treatment for hepatitis B is started, there are two main reasons for laboratory monitoring: (1) to evaluate for any medication-related complications to ensure the ongoing safety of the treatment and (2) to assess the effectiveness of treatment and to detect treatment failure if it occurs. This lesson will review the key elements of monitoring persons with chronic HBV, including persons who are not on therapy, persons who are receiving treatment, and persons who discontinue treatment.
- Module 2 Overview
Treatment of HBV - 0%Lesson 1
When to Initiate HBV TreatmentActivities- 0%Lesson 2
Choosing an Initial HBV Treatment RegimenLesson 3. Monitoring Persons On and Off HBV Therapy
Learning Objective Performance Indicators
- List the frequency and type of laboratory monitoring recommended for persons with chronic HBV who do not meet criteria for treatment initiation
- Review the timing, frequency and methods for liver disease staging in persons with chronic HBV
- Describe the initial and ongoing laboratory monitoring for persons receiving antiviral therapy for HBV
- Define a biochemical response, serologic response, and virologic response among persons receiving antiviral therapy for HBV
- Summarize the principles of HBV antiviral therapy discontinuation and in whom this can be considered
Last Updated: September 27th, 2023Author:H. Nina Kim, MD, MScH. Nina Kim, MD, MSc
Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneReviewers:Maria A. Corcorran, MD, MPH,Maria A. Corcorran, MD, MPH
Assistant Professor
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneDavid H. Spach, MDDavid H. Spach, MD
Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneTable of Contents- Monitoring Persons On and Off HBV Therapy
- Introduction
- Monitoring Persons with Chronic HBV Who are Not on Treatment
- Safety Monitoring in Persons with Chronic HBV on Treatment
- Definitions Used for Monitoring Response to Therapy
- Monitoring Treatment Response in Persons with Chronic HBV
- Overview of Monitoring Persons on Hepatitis B Treatment
- Discontinuation of Antiviral Therapy Based on Treatment Response
- Monitoring after Antiviral Therapy is Stopped
- Summary
- Citations
- Additional References
- Figures
- Tables
Introduction
Monitoring Persons with Chronic HBV Who are Not on Treatment
Persons with chronic HBV who do not meet the criteria for treatment initiation (e.g., inactive carriers or immune-tolerant patients) should continue to be monitored with serum ALT and HBV DNA levels at regular intervals to determine if they are transitioning into a more immune active state that might warrant antiviral therapy.[1,4,5] For a detailed discussion of indications for initiating HBV therapy, see the lesson When to Initiate HBV Treatment. In general, monitoring of ALT levels and HBV DNA is performed every 3 to 6 months in these untreated patients. Emphasizing the importance of regularly monitoring disease activity is a crucial aspect of counseling patients with chronic HBV and managing their expectations when they first seek care.
AASLD 2018 Hepatitis B Guidance
The 2018 AASLD Hepatitis B Guidance recommendations for laboratory monitoring vary depending on hepatitis B e antigen (HBeAg) status and the HBeAg status also impacts the ALT and HBV DNA thresholds used for clinical decision-making.[1] The following summarizes recommendations for monitoring in persons who do not have cirrhosis and are not on treatment, and these recommendations depend on HBeAg status, HBV DNA level, and the ALT relative to the upper limit of normal (ULN), which is considered 25 U/L for women and 35 U/Lfor men).
- HBsAg-Positive and HBeAg-Positive: In general, monitoring should consist of the HBV DNA levels and ALT levels every 3-6 months. In addition, persons with normal ALT levels and HBV DNA levels greater than 20,000 IU/mL should have HBeAg checked every 6-12 months. More frequent monitoring is indicated if the ALT level is elevated at baseline or the ALT becomes elevated during monitoring. Results from monitoring should inform decisions. If the ALT remains less than the ULN during the first year of monitoring, then the frequency of monitoring can be reduced to every 6 months. For example, persons who remain HBeAg positive with HBV DNA levels greater than 20,000 IU/mL after a 3- to 6-month period of elevated ALT levels greater than 2 times the upper limit of normal should be considered for antiviral treatment. In addition, persons who have persistently elevated HBV DNA levels (greater than 20,000 IU/mL) and slightly elevated ALT (less than 2 times the upper limit of normal) should be considered for liver biopsy.
- HBsAg-Positive and HBeAg-Negative: These individuals are considered to be in the inactive phase. For the first year, monitoring of ALT and HBV DNA should be conducted every 3 months and HBsAg once yearly to determine if the patient is indeed in the inactive phase. More frequent monitoring (and evaluation of potential causes) is indicated if the ALT level is persistently elevated or becomes elevated during monitoring. Results during monitoring should inform treatment decisions. If patients are stable with a consistently normal-range ALT for a minimum of 12 months, subsequent ALT testing can be extended to every 6 months.
Hepatitis B Primary Care Guidance
For persons with chronic hepatitis B who are not on treatment, the 2020 HBV Primary Care Workgroup Guidance recommends laboratory monitoring with serum ALT and HBV DNA levels every 6 months, and this does not vary by HBe antigen status.[6] More frequent testing is indicated if there is persistent ALT elevation above the upper limit of normal (>25 IU/L in women and >35 IU/L in men), which might indicate a transition to an immune-active phase that would warrant therapy.[6](Table 1)
Monitoring for Changes in HBV Antigen/Antibody Status
The loss of HBeAg (in patients who are HBeAg-positive at baseline) or the loss of HBsAg represent key immunologic milestones in patients with chronic HBV. Annual testing for HBsAg should be considered in patients who are HBeAg negative and have consistently normal ALT and low (<2,000 IU/mL) HBV DNA levels (e.g., inactive carriers), as HBsAg loss can occur spontaneously in these individuals.[1] Annual testing for HBeAg and anti-HBe should be considered in persons with immune-tolerant HBV infection (elevated HBV DNA but normal ALT), as these individuals may spontaneously lose their HBeAg-positive status.[1] Nevertheless, the cost-benefit utility of routine HBeAg, anti-HBe, and HBsAg serologic monitoring in untreated patients is unclear, particularly as HBsAg loss is considered a rare event, and HBeAg loss in patients not meeting HBV treatment criteria is unlikely to change management. Serologic testing of hepatitis B e antigen/antibody or surface antigen/antibody is considered optional in primary care settings.
Liver Disease Staging
The 2018 AASLD Hepatitis B Guidance also notes that liver disease severity should be assessed as part of the initial evaluation of patients with HBV.[1] Assessment of liver disease severity should include a baseline laboratory evaluation consisting of complete blood count (CBC) with platelet count, hepatic aminotransferases (ALT and AST), alkaline phosphatase, bilirubin, serum albumin, and prothrombin time at baseline.[1] In persons with viral hepatitis, ALT typically exceeds AST, but with advanced fibrosis, this ratio typically reverses. In addition to basic laboratory testing, there are several methods for staging liver fibrosis, which can be done to further evaluate liver disease in patients with HBV. When monitoring individuals not yet on therapy, the AASLD recommends that liver biopsy, or an alternative form of fibrosis assessment, be considered in patients with persistently elevated ALT that is borderline normal or mildly elevated (range of 1 to 2 times upper limit of normal: 25 IU/L in women, 35 IU/L in men), particularly if they acquired HBV at a very young age (as can occur in Asian countries where perinatal acquisition is the most common route for HBV acquisition) and if they are older than 40 years of age.[1] Patients who are found to have at least moderate to severe inflammation (Metavir score A3 or higher) or fibrosis (Metavir score F2 or higher) on histology are candidates for antiviral therapy.
-
Liver Biopsy: Liver biopsy has been, and remains, the gold standard for assessing liver disease severity in chronic HBV and is the only means by which we can assess both inflammatory activity and fibrosis. The risk of complications such as intrahepatic bleeding or biliary injury is low but can be up to 1.1% in patients with advanced liver disease.[7] Biopsy can also result in misclassification, particularly if there is under-sampling of liver tissue. Liver biopsy, however, can detect significant liver inflammation or fibrosis that is not always reflected in serum biomarkers and is therefore recommended in a subset of patients with chronic hepatitis B.[5]
- Transient Elastography: Transient elastography (FibroScan) is a noninvasive test that is performed using an ultrasound transducer probe to measure shear wave velocity, which correlates directly with liver stiffness. It has been shown to have reasonably good diagnostic performance for the detection of advanced fibrosis in a variety of patient settings, including HBV, and it is the preferred alternative method for assessing liver stiffness in patients with HBV when liver biopsy is not performed.[1,8]
- APRI and Fib-4:The AST-platelet ratio index (APRI) and Fibrosis-4 (FIB-4) index are formulae that predict the degree of underlying fibrosis in patients with viral hepatitis (see APRI and Fib-4 calculators). FIB-4 has been shown to have reasonable performance in differentiating mild (stage 0-1) from more advanced (stage 3-4) fibrosis in chronic HBV.[9,10] It should be noted, however, that these standard biomarkers are not sufficiently sensitive to detect advanced liver disease, particularly in patients with chronic HBV on antiviral therapy. In addition, these biomarkers do not adequately distinguish mild from moderate fibrosis. Because of this, repeated testing of CBC and the full hepatic function panel in an effort to calculate APRI and FIB-4 index scores is not warranted as part of monitoring most patients, particularly since changes in fibrosis would not be expected to occur rapidly or significantly enough to be reflected in these laboratory tests.
Hepatocellular Carcinoma (HCC) Screening
Hepatocellular carcinoma (HCC) screening is part of HBV monitoring and should occur when indicated, regardless of HBV treatment status.[1,4,11] For additional information on indications for HCC screening, please see the lesson Screening for Hepatocellular Carcinoma.
Safety Monitoring in Persons with Chronic HBV on Treatment
Safety Monitoring with Oral Antiviral Therapy
Oral antiviral therapy for HBV, using a first-line nucleoside analogue (entecavir) or nucleotide analogue (tenofovir alafenamide [TAF] or tenofovir disoproxil fumarate [tenofovir DF or TDF]), is generally considered safe, with minimal laboratory monitoring for safety needed, aside from renal safety monitoring in persons receiving tenofovir DF. The first-line oral antiviral agents, entecavir and tenofovir DF, require dose adjustment if the estimated creatine clearance is less than 50 mL/min. Further, since tenofovir DF has the potential to cause nephrotoxicity, most experts would avoid its use in persons with preexisting renal insufficiency (estimated creatine clearance less than 60 mL/min) and in persons with clinical features that are associated with a higher renal risk. In contrast, tenofovir alafenamide can be safely used for the treatment of HBV in persons with a creatinine clearance of greater than or equal to 15 mL/min or in persons receiving hemodialysis.
- Baseline Laboratory Studies: Prior to starting oral antiviral therapy, the following baseline laboratory studies are recommended:
- Complete blood count (CBC)
- Hepatic function panel that includes total bilirubin, ALT, AST, albumin and alkaline phosphatase
- Renal function panel; The renal assessment should include (1) a serum creatine to estimate creatinine clearance and calculate the glomerular filtration rate (GFR) and (2) evaluation of clinical risk factors for renal insufficiency. Preexisting renal insufficiency (GFR less than 60 mL/min) from any cause, including extrahepatic HBV-associated renal disease, would indicate an increased risk of tenofovir DF nephrotoxicity.
- HBV DNA levels with a highly sensitive quantitative assay should be ordered, as well as a hepatitis B e antigen (HBeAg) and hepatitis B e antibody (anti-HBe) if the status is not known.
- Baseline HIV test is recommended before treatment initiation—if the individual had undiagnosed HIV, use of monotherapy for HBV with entecavir, tenofovir alafenamide, or tenofovir DF would likely result in HIV drug resistance, due to the intrinsic HIV activity of these medications and the need to treat HIV with combination antiretroviral therapy.
- Evaluation for Renal Risk for Patients on Tenofovir DF: An assessment of renal risk should be made on an ongoing basis for those receiving tenofovir DF (TDF). The clinical factors associated with an increased risk for renal disease with TDF include decompensated cirrhosis, poorly controlled hypertension or diabetes, and the use of concurrent nephrotoxic medications. For persons receiving tenofovir DF, monitoring includes serum creatinine, serum phosphorus and urinalysis to assess urine glucose and proteinuria—the recommended interval of testing is recommended at least once annually and more frequently in patients at risk for renal injury or with preexisting renal impairment.[1] In cases of known or suspected TDF-associated nephrotoxicity, TDF should be discontinued and substituted with either TAF or entecavir, with appropriate consideration of prior treatment experience and drug resistance, before making this selection.
- Monitoring for Lactic Acidosis: The development of lactic acidosis is a rare complication of nucleoside/nucleotide therapy; this has been seen with both tenofovir DF and entecavir in patients with chronic HBV.[12,13] The mechanism for this is thought to be the drug’s inhibition of mitochondrial polymerase gamma inside the liver and muscle. It is a potentially fatal, life-threatening adverse effect that can manifest insidiously with non-specific symptoms such as fatigue, nausea, malaise, or abdominal discomfort. Decompensated cirrhosis, particularly with a MELD score greater than 20, and concurrent use of medications with additive mitochondrial toxicity are risk factors.[13] Routine laboratory monitoring for lactic acidosis is not necessary but assessment of the anion gap and testing of lactic acid level is warranted in a symptomatic patient with advanced liver disease.
Safety Monitoring with Peginterferon Therapy
Peginterferon-based therapy requires more frequent laboratory monitoring for safety and efficacy than oral antiviral therapy due to its unique set of toxicities. Adverse events with peginterferon can be diverse and range from initial flu-like symptoms to fatigue, nausea, diarrhea, anorexia with or without weight loss, emotional lability and depression, bone marrow suppression, and autoimmune phenomena such as thyroiditis. All persons receiving peginterferon should be monitored for these complications throughout the 48 weeks of therapy. In some individuals, these adverse effects can be severe enough to warrant dose reduction or discontinuation of therapy.
- Baseline Laboratory Studies: At baseline, a CBC, liver panel, renal function panel, HBV DNA level, HBeAg, anti-HBe, and thyroid stimulating hormone (TSH) level should be sent.
- Laboratory Monitoring on Treatment: Serum ALT should be assessed every 1-3 months during treatment to assess for hepatic flares since these often occur during interferon-based therapy for HBV. Laboratory monitoring should also include TSH every 3 months and a CBC with differential every 1-2 months, with the exact frequency determined by changes observed.
- Additional Monitoring on Therapy: Patients on peginterferon should be monitored at each visit for symptomatic anemia, as well as other complications, including neuropsychiatric disturbances in sleep or mood, infections, or autoimmune complications.
Definitions Used for Monitoring Response to Therapy
An appropriate understanding of virologic responses is required to assess treatment effectiveness in the context of both oral antiviral therapy and peginterferon-based therapy. The following are general definitions of terms describing the spectrum of treatment responses.[1,4,5,11]
Biochemical Responses (Based on ALT Levels)
- Biochemical Response: Persons on HBV treatment are considered to have a biochemical response to treatment if normalization of serum ALT level occurs while on treatment.
- Clinical Relapse: This occurs when a viral relapse is accompanied by or followed shortly by a rise in serum ALT to greater than 2 times the baseline ALT.
- Hepatitis Flare: A hepatitis flare is defined as an ALT increase of 3 times the baseline level and greater than 100 U/L.
Serologic Responses
A serologic response in a person with HBV who is receiving treatment can be described based on the dynamics of (1) HBeAg and anti-HBe or (2) HBsAg and anti-HBs.
- HBeAg/anti-HBe: A serologic response related to HBeAg and anti-HBe is defined as HBeAg loss and seroconversion to anti-HBe positive status for a person who is HBeAg-positive and anti-HBe negative at baseline. During this transition, typically there is typically a brief gap that occurs where the HBeAg has resolved, and anti-HBe has not yet appeared (Figure 2).
- HBsAg/anti-HBs (with gap): A serologic response related to HBsAg and anti-HBs is defined as HBsAg loss and seroconversion to anti-HBs positive status in a person who at baseline has a positive HBsAg and a negative anti-HBs. This occurs in only roughly 1% of persons per year on oral therapy. In some, a brief gap may occur during this transition where the HBsAg has resolved, but anti-HBs has not yet appeared (Figure 3).
- HBsAg/anti-HBs (without gap): A serologic response related to HBsAg and anti-HBs is defined as HBsAg loss and seroconversion to anti-HBs-positive status in a person who at baseline has positive HBsAg and negative anti-HBs. This occurs in only roughly 1% of persons per year on oral therapy. In some, the anti-HBs appears as the HBsAg is declining (Figure 4).
Virologic Responses (Based on HBV DNA)- Virologic Response (with Oral Antiviral Therapy): For persons receiving oral nucleoside analogue HBV therapy, a virologic response is defined as an undetectable HBV DNA level using a sensitive polymerase chain reaction (PCR)-based assay for viral quantitation, where the lower limit of detection is 10-12 IU/mL (Figure 5). Some expert panels suggest using a threshold of less than 60 IU/mL to signify a virologic response with oral nucleoside analogue therapy.
- Virologic Response (with Peginterferon Therapy): For persons receiving peginterferon for treatment of HBV, a virologic response is defined as the achievement of an HBV DNA level below 2,000 IU/mL (Figure 6).[14] Some experts have used 20,000 IU/mL as a higher threshold that defined virologic response with peginterferon therapy.
- Primary Virologic Response: A decline in HBV DNA of greater than 1 log10 IU/mL by 12 weeks of starting oral antiviral therapy (Figure 7).
- Primary Virologic Nonresponse: This is defined by a decline of HBV DNA level that is less than 1 log10 IU/mL at 12 weeks on oral antiviral therapy (Figure 8).
- Partial Virologic Response: This occurs when the decline of HBV DNA level is greater than 1 log10 IU/mL but still detectable at 24 weeks of oral antiviral therapy (in a person who is adherent with therapy) (Figure 9).
- Virologic Breakthrough: An increase of HBV DNA levels to greater than 1 log10 IU/mL compared with the lowest (nadir) HBV DNA level (Figure 10).
- HBV Reactivation: (1) a 2 log10 (100-fold) or more increase in HBV DNA level compared to baseline or (2) an HBV DNA level of at least 3 log10 (1,000) IU/mL in a patient previously undetectable (Figure 11).
- Viral Relapse: This refers to the emergence of an HBV DNA level greater than 2,000 IU/mL after stopping therapy in patients with a virologic response (Figure 12).
- Sustained Off-Treatment Virologic Response with Oral Therapy: For persons who discontinue oral antiviral therapy, this is defined as the presence of HBV DNA levels less than 2,000 IU/mL for at least 12 months after stopping therapy; this response is most likely to occur only after multiple years of oral therapy (Figure 13).
- Sustained Off-Treatment Virologic Response with Peginterferon Therapy: This describes the presence of HBV DNA levels less than 2,000 IU/mL for at least 12 months after stopping peginterferon treatment; the typical HBV treatment course with peginterferon therapy is 48 weeks (Figure 14).
Monitoring Treatment Response in Persons with Chronic HBV
Monitoring Treatment Response with Oral Antiviral Therapy
The cornerstone of monitoring the treatment responses in patients taking oral antiviral therapy is tracking the HBV DNA level. The degree to which HBV viral suppression is achieved and sustained during therapy has been shown to be a key predictor of clinical outcomes.[15,16] This is not surprising, given our understanding of HBV replication and the major role it plays in driving liver disease progression (both decompensated liver disease and hepatocellular carcinoma).[17,18] Ongoing monitoring of HBV DNA levels is needed to evaluate the initial treatment response and ensure early detection of virologic breakthrough and treatment failure. Treatment-emergent drug resistance can occur with oral antiviral therapy, although this is rare with the use of any of the three preferred oral antiviral therapy options (tenofovir DF, tenofovir alafenamide, and entecavir).
Monitoring HBV DNA Levels on Therapy
Most HBV guidelines recommend that HBV DNA quantification be ordered at baseline prior to treatment as well as at months 3 and 6 after initiating therapy.[1,4,5] Most persons who have consistent adherence to their treatment will achieve a decline in HBV DNA of greater than 1 log10 IU/mL by 12 weeks (primary virologic response) on one of the first-line nucleoside analogue agents (entecavir, tenofovir alafenamide, or tenofovir DF). If they do not, additional counseling on adherence and exploration of barriers to treatment is indicated. Following initiation of HBV therapy, the magnitude and timing of HBV viral decline can vary significantly. The degree and rapidity of the HBV DNA decline often depend on the person’s immunologic response against HBV, with those who are more immune active (i.e., higher baseline ALT) and less immune tolerant more likely to experience an efficient decline in HBV DNA levels.[19] Once a primary virologic response has been achieved, HBV DNA levels should continue to be monitored every 3 to 6 months. The 2018 AASLD Hepatitis B Guidance advocates for monitoring HBV DNA levels every 3 months until the HBV DNA becomes undetectable, particularly if the person on treatment is receiving an agent with a lower genetic barrier to resistance.[1]
- Time to Achieve Virologic Response: A virologic response, or achievement of complete HBV suppression to undetectable levels, is an important endpoint in nucleoside analogue therapy but can be delayed in many patients and often takes longer than 48 weeks, primarily due to the slower kinetics of HBV viral decline (compared with treatment-related declines in viral levels with HIV or HCV therapy).
- Approach to Persistent Viremia: Persistent viremia is defined as a leveling or plateau in the decline of HBV DNA and/or failure to achieve an undetectable HBV DNA level by 96 weeks of therapy. Persistent low-level viremia is the aforementioned, with a viral level of less than 2000 IU/ml but detectable at 96 weeks. Emerging data suggests this phenomenon of persistent low-level viremia may be associated with adverse clinical outcomes, namely accelerated fibrosis progression and increased risk for hepatocellular carcinoma.[20,21] The AASLD does not currently recommend augmenting or changing therapy in such cases, even in the setting of an elevated ALT level.[1]
- Approach to Virologic Breakthrough: Virologic breakthrough is defined as a 10-fold (1 log10 IU/ml) or greater increase in HBV DNA level in persons on treatment. This finding needs to be confirmed with a repeat HBV DNA level. This most often indicates a lapse in medication adherence or unanticipated medication interruption. Thus, the finding of a virologic breakthrough should first lead to an adherence evaluation, including a thorough review of pharmacy refill records, before pursuing further testing for HBV drug resistance. If good adherence is likely, then a virologic breakthrough should be considered. A virologic breakthrough can indicate the emergence of antiviral resistance mutations and, if left unchecked, can lead to the acquisition of additional mutations, further HBV viral replication, and, in some cases, hepatitis flares and hepatic decompensation.[22]
HBV Resistance Testing
In most settings, drug resistance is confirmed by sending an HBV genotypic resistance assay using consensus-based sequencing (similar to that used for HIV resistance testing) that is used to detect viral subpopulations bearing amino acid substitutions (mutations) in the reverse transcriptase region of the HBV genome known to confer drug resistance. The probability of resistance is influenced by a variety of clinical factors, including pretreatment HBV DNA level, prior treatment exposure, duration and potency of antiviral therapy, and, most importantly, the genetic barrier for resistance of the antiviral agent. The rates of HBV resistance are highest for lamivudine (65-70% at 4-5 years), intermediate for telbivudine (11 to 25% in 2 years), and adefovir (29% at 5 years), and lowest for entecavir in the absence of lamivudine resistance (2% at 5 years) and tenofovir (3% at 5 years) (Figure 15).[4,23,24] Management of drug resistance is a separate topic not covered in this discussion and should be done with expert consultation.
Monitoring Biochemical Response
A hepatic function panel (serum ALT, AST with or without total bilirubin, albumin, and alkaline phosphatase) is generally performed concurrently with the HBV DNA monitoring in persons on oral antiviral therapy. In most cases, the beginning of a biochemical response, as evidenced by the normalization of ALT and AST elevation (typically <25 U/L in women, <35 U/L in men), can be seen as early as a few months after the initiation of nucleoside analogue therapy in patients with immune-active disease, typically coincident with the decline in HBV DNA level.
Monitoring Serologic Response
- HBeAg Loss and anti-HBe Seroconversion: Serologic responses for HBe antigen should be evaluated in persons who are HBeAg positive at baseline to assess whether they lose HBeAg and subsequently develop anti-HBe. This represents an important immunologic milestone in treatment response and can signal greater intrahepatic control of HBV infection. It is also a benchmark for which consideration of treatment cessation can be made – see below for further discussion. The loss of HBeAg and seroconversion to anti-HBe occurs at variable rates with oral antiviral therapy and typically does not occur until virologic response is achieved. Thus, once a virologic response has been achieved, HBeAg and anti-HBe should be assessed every 6 months—until HBeAg loss and anti-HBe seroconversion occur.
- HBsAg Loss and anti-HBs Seroconversion: This seroconversion is the ultimate goal of any HBV antiviral therapy but is generally a rare event with oral antiviral therapy (Figure 16).[23,25,26,27,28] Nevertheless, evaluation of HBsAg and anti-HBs should be performed annually for those individuals who have achieved complete HBV viral suppression for more than a year, particularly since it may factor into decisions to stop therapy and assess for sustained off-treatment response. For persons who are HBeAg positive at baseline, monitoring for HBsAg loss and anti-HBs seroconversion should not start until HBeAg/Ab seroconversion has occurred. For persons who are HBeAg negative at baseline, evaluation for HBsAg and anti-HBs can be annually assessed once complete HBV suppression has been maintained for at least one year.
- Quantitative Hepatitis B Surface Antigen (HBsAg): The quantitative HBsAg reflects intrahepatic HBV viral burden that arises from integrated HBV DNA and can be measured via a commercial assay. This can have a role in predicting those who are likely to respond (or not) to peginterferon therapy, as well as those most likely to maintain sustained off-treatment control of their infection after cessation of oral antiviral therapy. Its exact role in monitoring remains uncertain at this time; AASLD 2018 guidance advises against routine use of this test.
Monitoring Treatment Response with Peginterferon Therapy
Peginterferon is recommended for a fixed duration of 48 weeks for chronic HBV infection, regardless of HBeAg status or other clinical factors. Peginterferon does not typically result in comparable on-treatment virologic responses as one might observe with oral antiviral therapy, but in selected patients, it can result in greater gains in control of HBV DNA levels and achieving serologic end points, even after treatment has been completed.[29] For example, in patients who are HBeAg negative, a 1-year course of peginterferon results in a higher rate of HBsAg loss than with the same duration of oral antiviral therapy (4% versus 0 to 1%) despite a lower rate of undetectable HBV DNA (63% versus 90 to 94%).[4] The desired treatment end points for peginterferon include sustained off-treatment HBV DNA less than 2,000 IU/mL, normalization of ALT, HBeAg loss (if HBeAg positive), and ultimately HBsAg loss. Monitoring of HBV DNA levels is recommended at baseline, 3 and 6 months after starting treatment, and at the end of treatment. Among the various biomarkers, quantitative serum HBsAg (qHBsAg) appears to be the strongest early on-treatment predictor of treatment response to peginterferon, and this biomarker has been used to guide early stopping of therapy in patients in Asia and Europe who have been identified as not likely not to respond.[1,4] Post-treatment monitoring for treatment response is important since many of the clinical end points noted above, particularly the serologic end points of HBeAg loss and seroconversion and HBsAg loss, may occur months or even years after cessation of peginterferon.
Overview of Monitoring Persons on Hepatitis B Treatment
Laboratory Monitoring with Oral Antiviral Therapy
The following table provides a summary overview of baseline monitoring and on-treatment monitoring in persons receiving oral antiviral therapy (Table 2).
Laboratory Monitoring While Receiving Oral Antiviral Therapy
The following table provides a summary overview of baseline monitoring and on-treatment monitoring in persons receiving peginterferon therapy (Table 3).
Discontinuation of Antiviral Therapy Based on Treatment Response
Oral antiviral agents have emerged as the main therapeutic option for chronic HBV treatment, given the favorable safety profile of these agents and the higher virologic response rates when compared with peginterferon-based therapy, where only an estimated 20 to 30% of patients achieve a sustained off-treatment virologic response.[30] The major oral agents recommended in clinical practice include the nucleoside analogue (entecavir) and the nucleotide analogues (tenofovir DF and tenofovir alafenamide). In the following discussion, the term nucleoside analogue will be used to refer to all of these oral agents. Oral antiviral therapy, however, can be costly, and the rates of functional cure or loss of HBsAg are low, even after years of therapy.[23] The necessity of prolonged therapy over an individual’s lifespan has generated interest in the potential for safe discontinuation of nucleoside/nucleotide analogue therapy and supervised treatment interruption.
Discontinuation of Therapy in Patients Who are HBeAg-Positive at Baseline
It is generally accepted and advised by most professional guidelines (AASLD, European Association for the Study of the Liver [EASL], Asian Pacific Association for the Study of the Liver [APASL]) that EASL, APASL) that individuals without cirrhosis who seroconvert from HBeAg-positive at baseline to anti-HBe-positive on therapy and experience sustained HBV suppression can discontinue nucleoside analogue therapy after a period of additional treatment, which is referred to as consolidation therapy.[1,4,11] The 2018 AASLD Hepatitis B Guidance recommends this consolidation be a minimum of 12 months of additional treatment with persistently normal ALT and undetectable serum HBV DNA,[1] but the optimal duration of consolidation therapy is not known.[14] Factors associated with viral relapse after nucleoside analogue discontinuation include high baseline HBV DNA level, lower baseline ALT, older age, male sex, and shorter treatment duration.[31] In addition, the rates of off-treatment virologic response in HBeAg-positive patients who have discontinued nucleoside analogues depend on the HBeAg status and time since discontinuation (Figure 17).[14]
Discontinuation of Therapy in Patients Who are HBeAg Negative at Baseline
Where there is more controversy and uncertainty—and where the guidelines diverge—is how to manage individuals who are HBeAg negative at baseline. Among individuals without cirrhosis, the EASL and APASL leave open the possibility of treatment discontinuation after a minimum of 2 years (APASL) or 3 years (EASL) of HBV DNA suppression.[4,11] In contrast, the AASLD advises against stopping nucleoside analogue therapy in HBeAg-negative patients; they recommend indefinite therapy unless there is a compelling clinical reason to stop.[1] They offer three general considerations for clinicians to weigh when considering nucleoside analogue discontinuation: (1) the risk of virologic relapse and events such as hepatic decompensation, liver cancer or death, (2) the burden of antiviral therapy for the individual (not only financial but medical) and (3) patient and provider preferences.[1]Additional considerations for treatment discontinuation
Another clinical scenario where discontinuation of nucleoside analogue therapy is considered is in those rare occasions when HBsAg is lost, with or without anti-HBs seroconversion. The AASLD notes the relative lack of evidence to guide treatment decisions for such persons. Recommendations for the reinitiation of therapy after nucleoside analogue discontinuation are not addressed by these guidelines, and decisions are largely left to the clinician to decide. The fear of HBV reactivation and viral relapse-associated hepatitis flare and disease decompensation has resulted in many patients either continuing therapy or restarting immediately at the first sign of HBV replication. Indeed, it is clear that a rebound of HBV viremia is nearly universal after nucleoside analogue discontinuation, although some individuals who experience a viral relapse can go on to have mild biochemical flares and then transition to an inactive carrier state with eventual loss of HBsAg.[31,32] If HBV transcriptional activity is silenced sufficiently to enable beneficial reexposure to HBV without the worry of dangerous hepatitis flares, and if HBV reexposure can potentially lead to long-lasting disease remission and even spontaneous HBsAg loss, then nucleoside analogue discontinuation may be an important therapeutic strategy in some individuals.[33] The challenge at the moment, and the reason why nucleoside analogue discontinuation is not the norm but the exception at this time, is that we do not have well-established biomarkers or clinical indicators to identify these individuals. It should be noted that due to the risk of morbidity and mortality associated with HBV reactivation, discontinuation of oral nucleoside or nucleotide analogue therapy is not recommended in patients with cirrhosis. A few cases of life-threatening viral relapse and deaths have been reported in patients with cirrhosis who stopped nucleoside or nucleotide analogue therapy.[34,35] There is general consensus across guidelines that two main prerequisites need to be met before treatment discontinuation can occur: (1) that close monitoring of disease activity is feasible (and acceptable to the patient) and (2) there is an absence of cirrhosis.Monitoring after Antiviral Therapy is Stopped
Monitoring after Antiviral Therapy is Stopped
The main reason for laboratory monitoring in patients who have received antiviral therapy for HBV is to monitor for HBV reactivation and flare in the setting of treatment discontinuation. Persons who stop treatment with either peginterferon or oral nucleoside (or nucleotide) therapy are at risk for HBV reactivation and associated hepatitis flare. Therefore, these individuals need regular monitoring after stopping therapy, particularly during the first year after treatment discontinuation. Recommended monitoring should include evaluation for HBV DNA level, ALT elevation, seroreversion (e.g., recurrent HBeAg after prior loss), and clinical decompensation (for example, ascites). The recommended frequency of monitoring is every 3 months for at least one year. More intensive monitoring is warranted for patients with cirrhosis: at monthly intervals for the first 6 months and then every 3 months thereafter for at least one year. In addition, when it comes to monitoring treatment response to peginterferon, post-treatment monitoring is as important since many of the clinical end points noted above, particularly the serologic end points of HBeAg loss and seroconversion and HBsAg loss, may occur months or even years after cessation of peginterferon.
Summary
- The HBV Primary Care Guidance recommends monitoring individuals with chronic HBV who do not warrant treatment with a serum ALT and HBV DNA level every 6 months.
- Screening for HCC, if indicated, should occur regardless of whether or not the patient is undergoing treatment and should be considered part of the monitoring and care of patients with chronic HBV.
- In addition to a complete blood count and hepatic and renal function panels, a baseline HIV test is indicated since monotherapy with entecavir, tenofovir alafenamide, or tenofovir DF would be contraindicated in persons with HIV.
- For persons receiving tenofovir DF, monitoring includes serum creatinine, serum phosphorus, and urinalysis to assess urine glucose and proteinuria; the recommended testing interval is at least once annually and more frequently in patients at risk for renal injury or with preexisting renal impairment.
- Routine laboratory monitoring for lactic acidosis is not necessary, but assessment of the anion gap and testing of lactic acid level is warranted in a symptomatic patient with advanced liver disease.
- Adverse events with peginterferon therapy can be diverse and can include flu-like symptoms, fatigue, nausea, diarrhea, anorexia (with or without weight loss), emotional lability, depression, bone marrow suppression, and autoimmune phenomena, such as thyroiditis. All patients receiving peginterferon should be monitored for these complications throughout the entire course of therapy.
- Ongoing monitoring of HBV DNA levels is needed to establish the initial treatment response to oral antiviral therapy and ensure early detection of a virologic breakthrough and treatment failure. This should be measured at baseline, then at 3 and 6 months to assess primary virologic response, which is a decline in HBV DNA of greater than 1 log10 IU/mL.
- Additional counseling on adherence and exploration of barriers to treatment is indicated if a patient does not achieve a primary virologic response on oral antiviral therapy.
- Once HBV viral suppression to undetectable levels has occurred on oral antiviral therapy, HBeAg and anti-HBe can be assessed every 6 months until HBeAg loss and anti-HBe seroconversion have occurred.
- Patients who stop either peginterferon or oral nucleoside analogue therapy are at risk for HBV reactivation and an associated hepatitis flare, and, therefore, need regular laboratory and clinical monitoring (typically every 3 months), particularly in the first year of stopping therapy.
Citations
- 1.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-99.[PubMed Abstract] -
- 2.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-55.[PubMed Abstract] -
- 3.McMahon BJ. Natural history of chronic hepatitis B. Clin Liver Dis. 2010;14:381-96.[PubMed Abstract] -
- 4.European Association For The Study Of The Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-98.[PubMed Abstract] -
- 5.Ghany MG. Current treatment guidelines of chronic hepatitis B: The role of nucleos(t)ide analogues and peginterferon. Best Pract Res Clin Gastroenterol. 2017;31:299-309.[PubMed Abstract] -
- 6.Tang AS, Thornton K, and HBV Primary Care Workgroup. Hepatitis B Management: Guidance for the Primary Care Provider. February 25, 2020.
- 7.Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877-83.[PubMed Abstract] -
- 8.Li Y, Huang YS, Wang ZZ, et al. Systematic review with meta-analysis: the diagnostic accuracy of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2016;43:458-69.[PubMed Abstract] -
- 9.Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30:546-53.[PubMed Abstract] -
- 10.Li J, Gordon SC, Rupp LB, et al. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J Viral Hepat. 2014;21:930-7.[PubMed Abstract] -
- 11.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-70.[PubMed Abstract] -
- 12.Jung TY, Jun DW, Lee KN, et al. Fatal lactic acidosis in hepatitis B virus-associated decompensated cirrhosis treated with tenofovir: A case report. Medicine (Baltimore). 2017;96:e7133.[PubMed Abstract] -
- 13.Lange CM, Bojunga J, Hofmann WP, et al. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology. 2009;50:2001-6.[PubMed Abstract] -
- 14.Papatheodoridis G, Vlachogiannakos I, Cholongitas E, et al. Discontinuation of oral antivirals in chronic hepatitis B: A systematic review. Hepatology. 2016;63:1481-92.[PubMed Abstract] -
- 15.Nguyen MH, Keeffe EB. Chronic hepatitis B: early viral suppression and long-term outcomes of therapy with oral nucleos(t)ides. J Viral Hepat. 2009;16:149-55.[PubMed Abstract] -
- 16.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-75.[PubMed Abstract] -
- 17.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73.[PubMed Abstract] -
- 18.Iloeje UH, Yang HI, Jen CL, et al. Risk and predictors of mortality associated with chronic hepatitis B infection. Clin Gastroenterol Hepatol. 2007;5:921-31.[PubMed Abstract] -
- 19.Zeuzem S, Gane E, Liaw YF, et al. Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. J Hepatol. 2009;51:11-20.[PubMed Abstract] -
- 20.Kim JH, Sinn DH, Kang W, et al. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology. 2017;66:335-43.[PubMed Abstract] -
- 21.Sun Y, Wu X, Zhou J, et al. Persistent Low Level of Hepatitis B Virus Promotes Fibrosis Progression During Therapy. Clin Gastroenterol Hepatol. 2020;18:2582-91.e6.[PubMed Abstract] -
- 22.Di Marco V, Marzano A, Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology. 2004;40:883-91.[PubMed Abstract] -
- 23.Buti M, Riveiro-Barciela M, Esteban R. Long-term safety and efficacy of nucleo(t)side analogue therapy in hepatitis B. Liver Int. 2018;38 Suppl 1:84-9.[PubMed Abstract] -
- 24.Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315-41.[PubMed Abstract] -
- 25.Seto WK, Lam YF, Fung J, et al. Changes of HBsAg and HBV DNA levels in Chinese chronic hepatitis B patients after 5 years of entecavir treatment. J Gastroenterol Hepatol. 2014;29:1028-34.[PubMed Abstract] -
- 26.Petersen J, Heyne R, Mauss S, et al. Effectiveness and Safety of Tenofovir Disoproxil Fumarate in Chronic Hepatitis B: A 3-Year Prospective Field Practice Study in Germany. Dig Dis Sci. 2016;61:3061-71.[PubMed Abstract] -
- 27.Marcellin P, Zoulim F, Hézode C, et al. Effectiveness and Safety of Tenofovir Disoproxil Fumarate in Chronic Hepatitis B: A 3-Year, Prospective, Real-World Study in France. Dig Dis Sci. 2016;61:3072-83.[PubMed Abstract] -
- 28.Ahn J, Lee HM, Lim JK, et al. Entecavir safety and effectiveness in a national cohort of treatment-naïve chronic hepatitis B patients in the US - the ENUMERATE study. Aliment Pharmacol Ther. 2016;43:134-44.[PubMed Abstract] -
- 29.Kao JH. HBeAg-positive chronic hepatitis B: why do I treat my patients with pegylated interferon? Liver Int. 2014;34 Suppl 1:112-9.[PubMed Abstract] -
- 30.Marcellin P, Bonino F, Lau GK, et al. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136:2169-79.[PubMed Abstract] -
- 31.Chang ML, Liaw YF, Hadziyannis SJ. Systematic review: cessation of long-term nucleos(t)ide analogue therapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Aliment Pharmacol Ther. 2015;42:243-57.[PubMed Abstract] -
- 32.Berg T, Simon KG, Mauss S, et al. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. J Hepatol. 2017;67:918-924.[PubMed Abstract] -
- 33.Berg T, Lampertico P. The times they are a-changing - A refined proposal for finite HBV nucleos(t)ide analogue therapy. J Hepatol. 2021;75:474-80.[PubMed Abstract] -
- 34.Jeng WJ, Chen YC, Chien RN, Sheen IS, Liaw YF. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2018;68:425-34.[PubMed Abstract] -
- 35.Lim SG, Wai CT, Rajnakova A, Kajiji T, Guan R. Fatal hepatitis B reactivation following discontinuation of nucleoside analogues for chronic hepatitis B. Gut. 2002;51:597-9.[PubMed Abstract] -
Additional References
- Villa E, Fattovich G, Mauro A, Pasino M. Natural history of chronic HBV infection: special emphasis on the prognostic implications of the inactive carrier state versus chronic hepatitis. Dig Liver Dis. 2011;43 Suppl 1:S8-14.[PubMed Abstract] -
- Wong GL, Seto WK, Wong VW, Yuen MF, Chan HL. Review article: long-term safety of oral anti-viral treatment for chronic hepatitis B. Aliment Pharmacol Ther. 2018;47:730-737.[PubMed Abstract] -
Figures
 Figure 1. Hepatitis B Disease PhasesThis illustrations shows the relationship between different hepatitis B immune phases and fluctuations in HBV DNA and serum alanine aminotransferase (ALT) levels.Illustration: David H. Spach, MD
Figure 1. Hepatitis B Disease PhasesThis illustrations shows the relationship between different hepatitis B immune phases and fluctuations in HBV DNA and serum alanine aminotransferase (ALT) levels.Illustration: David H. Spach, MD Figure 2. On-Treatment: HBeAg/anti-HBe Serologic Responses (with gap)Illustration: David H. Spach, MD
Figure 2. On-Treatment: HBeAg/anti-HBe Serologic Responses (with gap)Illustration: David H. Spach, MD Figure 3. On-Treatment: HBsAg/anti-HBs Serologic Response (with gap)Illustration: David H. Spach, MD
Figure 3. On-Treatment: HBsAg/anti-HBs Serologic Response (with gap)Illustration: David H. Spach, MD Figure 4. On-Treatment: HBsAg/anti-HBs Serologic Response (without gap)Illustration: David H. Spach, MD
Figure 4. On-Treatment: HBsAg/anti-HBs Serologic Response (without gap)Illustration: David H. Spach, MD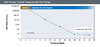 Figure 5. Virologic Response (with Oral Therapy)Illustration: David H. Spach, MD
Figure 5. Virologic Response (with Oral Therapy)Illustration: David H. Spach, MD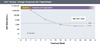 Figure 6. Virologic Response (with Peginterferon Treatment)Illustration: David H. Spach, MD
Figure 6. Virologic Response (with Peginterferon Treatment)Illustration: David H. Spach, MD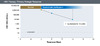 Figure 7. Primary Virologic ResponseIllustration: David H. Spach, MD
Figure 7. Primary Virologic ResponseIllustration: David H. Spach, MD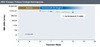 Figure 8. Primary Virologic NonresponseIllustration: David H. Spach, MD
Figure 8. Primary Virologic NonresponseIllustration: David H. Spach, MD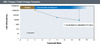 Figure 9. Partial Virologic ResponseIllustration: David H. Spach, MD
Figure 9. Partial Virologic ResponseIllustration: David H. Spach, MD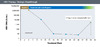 Figure 10. Virologic BreakthroughIllustration: David H. Spach, MD
Figure 10. Virologic BreakthroughIllustration: David H. Spach, MD Figure 11. HBV ReactivationIllustration: David H. Spach, MD
Figure 11. HBV ReactivationIllustration: David H. Spach, MD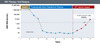 Figure 12. VIral RelapseIllustration: David H. Spach, MD
Figure 12. VIral RelapseIllustration: David H. Spach, MD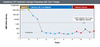 Figure 13. Sustained Off-Treatment Virologic Response with Oral TherapyIllustration: David H. Spach, MD
Figure 13. Sustained Off-Treatment Virologic Response with Oral TherapyIllustration: David H. Spach, MD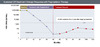 Figure 14. Sustained Off-Treatment Virologic Response with Oral TherapyIllustration: David H. Spach, MD
Figure 14. Sustained Off-Treatment Virologic Response with Oral TherapyIllustration: David H. Spach, MD Figure 15. Cumulative Incidence of HBV Resistance with Oral Antiviral AgentsAbbreviations: ND = no dataSource: European Association For The Study Of The Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-98. Reproduced with permission from Journal of Hepatology [https://www.journal-of-hepatology.eu]
Figure 15. Cumulative Incidence of HBV Resistance with Oral Antiviral AgentsAbbreviations: ND = no dataSource: European Association For The Study Of The Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-98. Reproduced with permission from Journal of Hepatology [https://www.journal-of-hepatology.eu] Figure 16. HBV Oral Antiviral Therapy: Cumulative Incidence HBV DNA Suppression and HBsAg ClearanceAbbreviations: TDF = tenofovir disoproxil fumarate; ETV = entecavirSource: (France) Marcellin P, Zoulim F, Hézode C, et al. Effectiveness and Safety of Tenofovir Disoproxil Fumarate in Chronic Hepatitis B: A 3-Year, Prospective, Real-World Study in France. Dig Dis Sci. 2016;61:3072-83. (Germany) Petersen J, Heyne R, Mauss S, et al. Effectiveness and Safety of Tenofovir Disoproxil Fumarate in Chronic Hepatitis B: A 3-Year Prospective Field Practice Study in Germany. Dig Dis Sci. 2016;61:3061-71. (United States): Ahn J, Lee HM, Lim JK, et al. Entecavir safety and effectiveness in a national cohort of treatment-naïve chronic hepatitis B patients in the US - the ENUMERATE study. Aliment Pharmacol Ther. 2016;43:134-44. (China) Seto WK, Lam YF, Fung J, et al. Changes of HBsAg and HBV DNA levels in Chinese chronic hepatitis B patients after 5 years of entecavir treatment. J Gastroenterol Hepatol. 2014;29:1028-34.
Figure 16. HBV Oral Antiviral Therapy: Cumulative Incidence HBV DNA Suppression and HBsAg ClearanceAbbreviations: TDF = tenofovir disoproxil fumarate; ETV = entecavirSource: (France) Marcellin P, Zoulim F, Hézode C, et al. Effectiveness and Safety of Tenofovir Disoproxil Fumarate in Chronic Hepatitis B: A 3-Year, Prospective, Real-World Study in France. Dig Dis Sci. 2016;61:3072-83. (Germany) Petersen J, Heyne R, Mauss S, et al. Effectiveness and Safety of Tenofovir Disoproxil Fumarate in Chronic Hepatitis B: A 3-Year Prospective Field Practice Study in Germany. Dig Dis Sci. 2016;61:3061-71. (United States): Ahn J, Lee HM, Lim JK, et al. Entecavir safety and effectiveness in a national cohort of treatment-naïve chronic hepatitis B patients in the US - the ENUMERATE study. Aliment Pharmacol Ther. 2016;43:134-44. (China) Seto WK, Lam YF, Fung J, et al. Changes of HBsAg and HBV DNA levels in Chinese chronic hepatitis B patients after 5 years of entecavir treatment. J Gastroenterol Hepatol. 2014;29:1028-34. Figure 17. Virologic Responses after Discontinuation of Nucleoside or Nucleotide Analogue TherapyAbbreviation: NA = nucleoside or nucleotide analogueSource: Papatheodoridis G, Vlachogiannakos I, Cholongitas E, et al. Discontinuation of oral antivirals in chronic hepatitis B: A systematic review. Hepatology. 2016;63:1481-92.
Figure 17. Virologic Responses after Discontinuation of Nucleoside or Nucleotide Analogue TherapyAbbreviation: NA = nucleoside or nucleotide analogueSource: Papatheodoridis G, Vlachogiannakos I, Cholongitas E, et al. Discontinuation of oral antivirals in chronic hepatitis B: A systematic review. Hepatology. 2016;63:1481-92.Tables
Table 1. HBV Primary Care Workgroup—Hepatitis B Management: Guidance for the Primary Care ProviderManagement of the HBsAg+Patient1
HBV DNA ALT (U/L) Management For people with Cirrhosis Any
Any - TREAT with antiviral medication
- Monitor HBV DNA and ALT every 6 months
- Refer to a specialist for screening endoscopy and, if needed, for other cirrhosis-related complications
- Hepatocellular (HCC) surveillance, including in persons who become HBsAg(-)
- All patients with decompensated cirrhosis2 should be promptly referred to a hepatologist
For people without Cirrhosis >2,000 IU/mL
Elevated3 - TREAT with antiviral medication
- Monitor HBV DNA and ALT every 6 months
- Monitor HBeAg and anti-HBe every 6 months in patients who are HBeAg+ at time of treatment initiation to evaluate for seroconversion from HBeAg(+)/anti-HBe(-) to HBeAg(-)/anti HBe(+)
- Check HBsAg annually if/when HBeAg negative
Normal - Monitor HBV DNA and ALT every 6 months
- Liver fibrosis assessment every 2 to 3 years
≤2,000 IU/mL Elevated3 - Evaluate other etiologies for elevated ALT
- Monitor HBV DNA and ALT every 6 months
Normal - Monitor HBV DNA and ALT every 6 months and
- HBsAg every 1 year for seroclearance
Abbreviations: ALT = alanine aminotransferase
1 In contrast to other HBV guidelines that have incorporated HBeAg status into treatment initiation decisions for non-cirrhotic HBsAg(+) patients, this guidance for primary care providers uses only HBV DNA and ALT to determine initial treatment indication in non-cirrhotic HBsAg(+) patients.
2 Patients should be considered to have decompensated cirrhosis and promptly referred to a hepatologist if any of the following are present: jaundice, ascites, variceal hemorrhage, hepatic encephalopathy, or a Child-Turcotte-Pugh (CTP) score ≥7 (see Hepatitis B Online CTP calculator.
3 Elevated ALT defined as >25 U/L in females and >35 U/L in males that is persistent for at least 3 to 6 months.Source:- Tang AS, Thornton K, and HBV Primary Care Workgroup. Hepatitis B Management: Guidance for the Primary Care Provider. February 25, 2020. [HBV Primary Care Workgroup]
Table 2. Treatment of HBV: Baseline and On-Treatment Monitoring with Oral Antivival Therapy
BASELINE MONITORING - Chemistry Panel including serum creatinine, hepatic panel (total bilirubin, ALT, AST, albumin, and alkaline phosphatase)
- Complete blood count with platelet count
- Hepatitis B e antigen (HBeAg) and Hepatitis B e antibody (anti-HBe)
- HBV DNA (quantitative)
- Coinfection screening
- HIV-1/2 antigen-antibody immunoassay
- Hepatitis C antibody
- Hepatitis A antibody)
MONITORING ON TREATMENT - Hepatic function panel (or ALT alone) at minimum every 3 to 6 months
- If on tenofovir DF (every 12 months (and more frequently in persons with risks for nephrotoxicity)
- Serum creatinine, serum phosphorus, and
- Urinalysis
- HBV DNA (every 3 months while detectable and every 6 months thereafter)
Table 3. Treatment of HBV: Baseline and On-Treatment Monitoring with Peginterferon Therapy
BASELINE MONITORING - Chemistry Panel: including serum creatinine, hepatic panel (total bilirubin, ALT, AST, albumin, alkaline phosphatase)
- Complete blood count with differential and platelet count
- Hepatitis B e antigen (HBeAg) and Hepatitis B e antibody (anti-HBe)
- HBV DNA (quantitative)
- Coinfection screening
- HIV-1/2 antigen-antibody immunoassay
- Hepatitis C virus (HCV) antibody with reflex to H
- Hepatitis A antibody [assess for hepatitis A immunization])
- Thyroid stimulating hormone (TSH)
MONITORING ON TREATMENT - Hepatic function panel (or ALT alone) at minimum every 1 to 3 months
- Complete blood count with differential every 1 to 2 months
- Thyroid stimulating hormone (TSH) every 3 months
- HBV DNA (every 3 months, 6 months, and end of treatment
- Optional: quantitative HBsAg
Share by e-mail
Check
-On-
Learning
QuestionsThe Check-on-Learning Questions are short and topic related. They are meant to help you stay on track throughout each lesson and check your understanding of key concepts.You must be signed in to customize your interaction with these questions.
- 0%Lesson 2
- HBV Medications
- Self-Study Modules CNE/CME
- HBV Epidemiology
- HBV Screening, Testing, and Diagnosis
- HBV Immunizations
- Initial Evaluation of Persons with Chronic Hepatitis B
- When to Initiate HBV Treatment
- Choosing an Initial HBV Treatment Regimen
- Monitoring Persons On and Off HBV Therapy
- Preventing HBV Perinatal Transmission
- Screening for Hepatocellular Carcinoma
- Occupational HBV Postexposure Prophylaxis
- Hepatitis B Reactivation in the Setting of Immunosuppression
Quick Reference
- HBV Epidemiology
- HBV Screening, Testing, and Diagnosis
- HBV Immunizations
- Initial Evaluation of Persons with Chronic Hepatitis B
- When to Initiate HBV Treatment
- Choosing an Initial HBV Treatment Regimen
- Monitoring Persons On and Off HBV Therapy
- Preventing HBV Perinatal Transmission
- Screening for Hepatocellular Carcinoma
- Occupational HBV Postexposure Prophylaxis
- Hepatitis B Reactivation in the Setting of Immunosuppression
- Clinical ChallengesHepatitis B Primary Care Guidance Master Bibliography
Contributors - Tools & Calculators
Since you've received 80% or better on this quiz, you may claim continuing education credit.
You seem to have a popup blocker enabled. If you want to skip this dialog please Always allow popup windows for the online course.























